Geoscience Reference
In-Depth Information
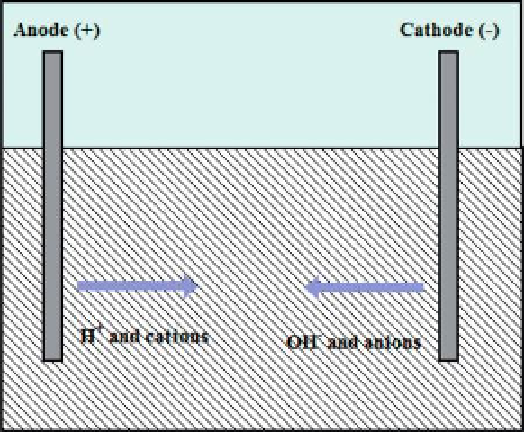
Figure 1.6.
Movement of ions in EK.
results in a temporary acidification of the soil zone that is treated. However, it is not possible to
theoretically predict the time required to establish equilibrium. The mobility of the hydronium
cation is about two times higher than that of the hydroxide ion, and significantly higher than
that of metal ions. However, in the soil, the hydronium cation is rapidly sorbed at the available
ion exchange sites where the metal ions are in turn released to the electrolyte. At the same
time, there is a similar movement, but slower and in the opposite direction, of hydroxide anions
from the cathode to the anode, desorbing anions attached to the soil particles. The encounter of
metal cations with hydroxide anions (since they are moving in opposite directions) may cause
precipitation. In practice, however, this is avoided by making the catholyte slightly acidic.
The main factors to consider in designing a facility for As removal by electrokinetics are as
follows:
•
Creation of anode and cathode compartments through ion permeable housing (anionic or
cationic membranes) with a diameter of 100-120 mm. These are placed in the contaminated
medium and connected to a centralized management system of electrolytes. Each housing
contains an electrode. The system is built with alternate rows of anodes and cathodes. The
electrolytes are circulated in a closed circuit between the electrode sheaths and the management
system of electrolytes (MSE). Through these electrolytes, the pH is maintained at a specified
value.
•
A potential difference is then applied to the electrodes. The water undergoes electrolysis at the
electrodes, forming H
+
and O
2
, which is generated at the anode, and OH
−
and H
2
, generated at
the cathode. These ions migrate from the electrodes housing to the soil creating a considerable
(but temporary) variation of the pH. This allows the desorption of the ionic contaminants. As
such, it is not necessary to inject acid to the subsurface.
•
Once desorbed, the contaminant ions migrate to the respective electrodes under the action of
and the cations to the cathodes. They flow through the housings of the electrodes and enter the
electrolytes circulation system.
•
The critical point of the management system is the careful control of pH and other conditions
of the electrolyte in the electrodes housing;
•
The contaminants are recovered from the solution obtained from the circulation of the
electrolytes.
Search WWH ::

Custom Search