Geoscience Reference
In-Depth Information
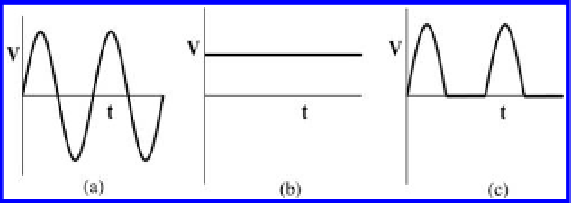
Figure 5.4. Types of electricity. (a) alternating current (AC), (b) full-wave direct current (DC), and
(c) half-wave direct current (DC).
the contaminants are removed by the mechanism of soil heating rather than the transport mech-
anism. Compared with the full-wave DC, the half-wave DC can facilitate the transformation
process of the contaminants, and promote solubilization or mobilization of contaminants due to
its pulse effect. If contaminants initially exist as strongly bound fractions, the half-wave DC is
favorable at the early stage of the process. After the application of the half-wave DC, the full-wave
DC is supplied to transport the dissolved or soluble forms of contaminants. Except for three types
of electricity, pulsed electricity has been applied to enhance the electrokinetic process (Ryu
et al
.,
2009). Pulsed electricity can improve desorption and dissolution of contaminants and finally
increase the overall efficacy of the process. Depending on the chemical forms of contaminants
and the electrical features of the soil media, electricity can be optimized by selection of sole type
or coupling of different types.
5.4
FIELD APPLICATIONS OF ELECTROKINETIC REMEDIATION
A number of studies on electrokinetic remediation have been undertaken by researchers using var-
ious contaminated sources, enhancement schemes, and monitoring and assessment techniques.
The representative research is presented in this section, which focuses on electrokinetic remedia-
tion of soils contaminated with inorganic contaminants, such as metals, heavy metals, arsenic, and
fluorine. Prior to introducing field applications, the laboratory- or pilot-scale studies performed
netic removal of contaminants from various types of soils, sediments, tailings, and sludges.
In addition, several enhancement schemes have been evaluated to improve the performance of
electrokinetic remediation because the most important aspect in the removal of heavy metals
from a solid matrix is their mobilization. Various enhancing reagents have been tested on elec-
trolyte or pretreatment solutions, and some elucidated different effects and trends depending on
the different metal species, even under equivalent conditions. Reddy and his colleagues con-
ducted a number of investigations to evaluate many chemical reagents, such as acetic acid, citric
acid, EDTA (ethylenediaminetetraacetic acid), DTPA (diethylene triamine penta acetic acid), KI
(potassium iodide), HPCD (hydroxypropyl-
-cyclodextrin), H
2
SO
4
, NaOH and NaCl as elec-
trolyte solutions, and humic acid, ferrous iron and sulfide as reducing agents, to remove mixed
heavy metals (Cr, Ni and Cd) from kaolin and glacial till soils (Al-Hamdan and Reddy, 2006;
Reddy and Ala, 2005; Reddy and Chinthamreddy, 2003; Reddy
et al
., 2001a; 2001b; 2004).
The technique using ion exchange membranes for electrokinetics is referred to as electrodia-
lytic remediation. When an electrokinetic treatment is applied without any conditioning, metals
may precipitate as hydroxides near the cathode region where the pH is increased, resulting in a
decreased removal efficiency. A popular enhancement scheme to prevent the hydroxide precipi-
tates is to apply an ion exchange membrane to the electrode compartments (Gardner
et al
., 2007;
Hansen
et al
., 1997; Kim
et al
., 2005a; Li
et al
., 1998; Nystroem
et al
., 2005; Ottosen
et al
.,
2003; Pedersen
et al
., 2005; Ribeiro
et al
., 2000). Many researchers have suggested numerous
techniques to predict and evaluate process efficiency as well. Because the initial chemical forms of
β
Search WWH ::

Custom Search