Geoscience Reference
In-Depth Information
composition of a terrestrial planet can therefore
be discussed with some confidence.
The composition of the Sun, meteorites,
comets, interplanetary dust particles and other
planets provide information that may be use-
ful in deducing the overall composition of our
planet, most of which is inaccessible to direct
observation. Compilations of solar and cosmic
abundances agree fairly closely for the more sig-
nificant rock-forming elements. From these data
simple models can be made (Tables 3.8, 3.9) of
the Earth's bulk chemical composition and min-
eralogy, assuming that the mantle is completely
oxidized. The composition in the first column of
Table 3.9 is based on cosmic abundances. This is
converted to weight fractions via the molecular
weight and renormalization.
The Fe
2
O requires some comment. Based
on cosmic abundances, it is plausible that the
Earth's core is mainly iron; however, from seis-
mic data and from the total mass and moment
of inertia of the Earth, there must be a light alloy-
ing element in the core. Of the candidates that
have been proposed (O, S, Si, N, H, He and C), only
oxygen and silicon are likely to be brought into
a planet in refractory solid particles -- the others
are very volatile elements and will tend to be con-
centrated near the surface or in the atmosphere
or lost to space. The hypothetical high-pressure
phase Fe
2
O has about the right density to match
core values. If most of the iron is in the core, in
Fe
2
O proportions, then the mass of the core will
be 30--34 weight% of the planet. The actual mass
of the core is 33%. There may also be some sulfur,
carbon, and so on in the core, but little or none
seems necessary.
Since most of the volume of a terrestrial
planet is oxygen, the oxygen isotopes of candi-
date materials play a key role in deciding what
to assemble a planet from. Oxygen isotopes imply
that the Earth is made of enstatite meteorites
or a mixture of meteorites that bracket the iso-
topic composition of the Earth and these mete-
orites. The bulk oxygen-isotopic composition of
the Earth precludes more than a few percent
of carbonaceous chondritic material accreting to
the Earth.
When the mantle is referred to as having the
composition of CI chondrites, or 'chondritic,' it is
+
2.0
Eclogite
+
1.0
Oceanic
Crust
Primitive
Picrite
AVER.
MANTLE
0
Peridotite
Pyrolite
−
1.0
SiO
2
TiO
2
AI
2
O
3
Major oxide
FeO
MgO
CaO
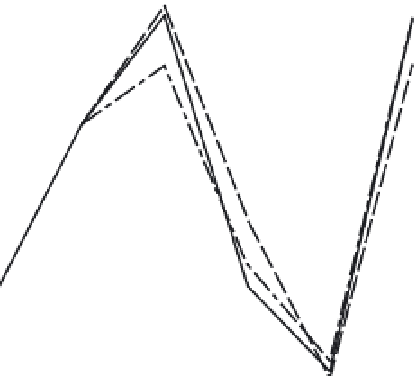
Fig. 3.1
Bulk chemistry of ultramafic rocks (peridotite) and
basic, or basaltic, rocks (oceanic crust, picrite, eclogite)
normalized to average mantle composition based on
cosmochemical considerations and an assumption about the
FeO content of the mantle. Pyrolite is a hypothetical upper
rock but it has been proposed to be representative of the
whole mantle. If so, the Mg/Si ratio of the mantle will not be
chondritic. A composition equivalent to 80% peridotite and
20% eclogite (or basalt), shown by triangles, is a mix that
reconciles petrological and cosmochemical major-element
data. Allowance for trace-element data and a possible
MgSiO
3
-rich lower mantle reduces the allowable basaltic
component to 15 weight% or less.
then the planets would have formed inhomoge-
nously. As a planet grows, the gravitational
energy of accretion increases, and impact vapor-
ization becomes more important for the larger
planets and for the later stages of accretion. The
assumption that Earth has cosmic abundances
of the elements is therefore only a first approx-
imation but is likely to be fairly accurate for
the involatile elements. There is little dispersion
of the refractory elements among the various
stony meteorite classes, suggesting that these ele-
ments are not appreciably fractionated by pre-
accretional processes. Fortunately, the bulk of a
terrestrial planet is iron, magnesium, silicon, cal-
cium, and aluminum and their oxides. The bulk