Geoscience Reference
In-Depth Information
2500
Liquid
Majorite
Perov-
skite
2000
3 PEROVSKITES
280
High
Clinoenstatite
Orthoenstatite
Protoenstatite
Ilmenite
Modified
Spinel
1500
ilm
+
Al
2
O
3
+
High-P
Clinoenstatite
240
Ca
−
pv
+
+
Stishovite
2 pv
gt
−
mj
MgO
+
gt
−
mj
1000
Low
Clinoenstatite
ilm
Spinel
+
Stishovite
+
MgO
b
+
mj s.s.
gt
200
di
g
500
5
10
15
20
25
b
OL
+
OP
+
GT
ILMENITE
Pressure, GPa
160
olivine
pyroxene
+
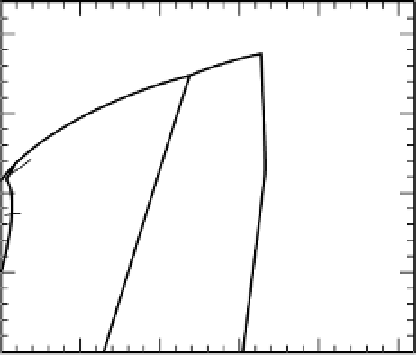
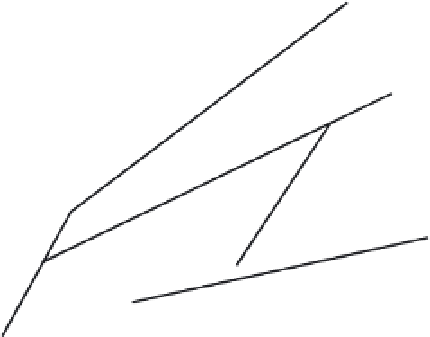
Fig. 22.5
Phase relations in MgSiO
3
synthesized from
a large number of studies (Presnall, 1995).
+
garnet
b
ENSTATITE
120
a
garnet with increasing pressure and eventually
a homogenous garnet solid solution is formed.
When orthopyroxene is also present, the gar-
net and clinopyroxene compositions move toward
orthopyroxene with increasing pressure; that is,
the orthopyroxene component dissolves in both
the garnet and the clinopyroxene and eventually
disappears. At this point an MgSi-rich, aluminum-
deficient garnet coexists with a magnesium-
rich diopside. Garnet then moves toward the
clinopyroxene composition as diopside dissolves
in the garnet. Therefore, in contrast to the
bimineralic eclogite system, the garnet takes a
detour toward MgSiO
3
before it heads toward
CaMgSi
2
O
6
. In either case, orthopyroxene dis-
appears at a relatively low pressure. A synthe-
sis is given in Figure 22.5. Note that the high-
temperature sequence of transitions is different
from the low-temperature sequence. The result-
ing densities and seismic velocities are also quite
different. The low-temperature minerals (
spinel
1000
2000
3000
Temperature (K)
Fig. 22.6
Equilibrium phase boundaries for mantle minerals.
Dashed lines are olivine system boundaries; solid lines are
pyroxene--garnet system boundaries. Approximate adiabats
are shown for 'normal' mantle and 'cold slab' mantle. Note
the different phase assemblages, at constant pressure, for the
two adiabats.
garnet solid-solution field occurs between
enstatite,
ilmenite
and
perovskite
. Diopside and
jadeite, components of clinopyroxene, are stable
to higher pressures than enstatite. Diopside
collapses to a dense calcium-rich phase, (Ca--pv),
at pressures less than required to transform
enstatite to
perovskite
. Garnet (gt) itself is stable
throughout most of the upper mantle, although
it dissolves pyroxene at high pressure. Note
that the phase assemblages along the 'cold
slab' adiabat are different from those along the
'normal mantle' adiabat at almost all pressures;
this
+
stishovite,
ilmenite
) are 10% to 20% higher in veloc-
ity than the high-temperature minerals (pyrox-
ene, majorite).
is
true
for
temperature
contrasts
much
◦
C chosen for purposes of
smaller than the 800
illustration.
Temperature variations in a convecting man-
tle are expected to fluctuate by about 100 degrees
on both sides of the 'normal' temperature. Tem-
peratures in a homogenous self-compressed solid
or
The CMAS system
The system CaO--MgO--Al
2
O
3
--SiO
2
(CMAS) is
shown in Figure 22.6 in simplified form. High
temperatures and the presence of Al
2
O
3
stabilize
the majorite (mj) structure, and a broad majorite-
a
vigorously
convecting
homogenous
fluid