Geoscience Reference
In-Depth Information
potassium, 0.06 ppm uranium and 0.27 ppm
thorium. The basaltic components of pyrolite
have much higher concentrations of these and
other LIL. Refractory ratios such as U/Ca and
Th/Ca for pyrolite are about 50% higher than
chondritic because of the high LIL content of
the arbitrarily chosen basalt. One disadvantage of
two-component pyrolite-type models is that the
final results are controlled by the arbitrary choice
of components, including basalt, and composi-
tions. Indeed, the various pyrolite models differ
by an order of magnitude in, for example, the
abundance of potassium.
By and large, there is excellent agreement
with cosmochemical models (Table 13.3) and BSE
found by the above inversion. In the present
model, the alkalis lithium, potassium, rubidium
and cesium are somewhat more depleted than
in M&A, as are volatiles such as chlorine, vana-
dium and cadmium. The Rb/Sr and K/U ratios are
correspondingly reduced. The elements that are
excessively depleted (P, S, Fe, Co, Ni, Ge, Se, Ag,
Re, Os, Ir and Au) are plausibly interpreted as
residing in the core. Note that the chalcophiles
are not all depleted. In particular, lead is not
depleted relative to other volatiles such as man-
ganese, fluorine and chlorine, which are unlikely
to be concentrated in the core. The composi-
tion of primitive mantle, derived by the above
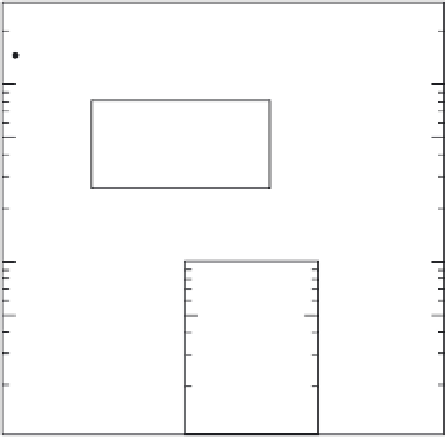
approach, is given in Figure 13.2.
Some elements are extraordinarily concen-
trated into the crust. The above results give the
following proportions of the total mantle-plus-
crust inventory in the continental crust; rubid-
ium, 58%; cesium, 53%; potassium, 46%; barium,
37%; thorium and uranium, 35%; bismuth, 34%;
lead, 32%; tantalum, 30%; chlorine, 26%; lan-
thanum, 19% and strontium, 13%.
In addition, the atmospheric
40
Ar content rep-
resents 77% of the total produced by 151 ppm
potassium over the age of the Earth. This has
probably degassed from the crust and upper man-
tle and probably reflects 23% retention, rather
than 23% primordial undegassed mantle. These
results all point toward an extensively differen-
tiated Earth and efficient upward concentration
of the incompatible trace elements. It is difficult
to imagine how these concentrations could be
achieved if the bulk of the mantle is still prim-
Zr
Nb
Mg
Al
Si
Sc
Ti
La
Nd
Sm
Eu
Tb
Yb
Lu
Ta
Co
Sr Y
Ba
Th
U
Ce
V
Li
1.0
Cr
Refractories
700 - 1300 K
Volatiles
Chalcophile
Siderophile
< 700 K
Mn
Ga
Na
Sn
Fe
Cu
K
Co
Ni
Rb
0.01
0.1
Zn
Cl
P
In
Cs
Re
OsIr
Tl
Pb
Cd
S
Ge
Au
Bi
Ag
Se
0.01
0.001
Fig. 13.2
Abundances of elements in 'primitive mantle'
(mantle
+
crust) relative to C1, derived by mixing mantle
components to obtain chondritic ratios of the refractory
lithophile elements.
itive or unfractionated. If only the upper man-
tle provides these elements to the crust, one
would require more than 100% removal of most
of the LIL (U, Th, Bi, Pb, Ba, Ta, K, Rb and Cs).
More likely, the whole mantle has contributed to
crustal, and upper-mantle, abundances, and most
of the mantle is strongly depleted and, probably,
infertile. The crust, MORB reservoir and the Q
component account for a large fraction of the
incompatible trace elements. It is likely, there-
fore, that the lower mantle is depleted in these
elements, including the heat producers potas-
sium, uranium and thorium.
In an alternative approach we can replace
UMR and OPX by their primary constituent min-
erals, olivine, orthopyroxene and clinopyroxene.
The present mantle can then be viewed as a five-
component system involving olivine, orthopyrox-
ene, clinopyroxene, MORB (cpx and gt) and Q.
In this case the LIL inventory of the primitive
mantle is largely contained in four components:
MORB, Q, clinopyroxene and CRUST. The results
of this approach are: olivine, 33%, orthopyrox-
ene, 48.7%, clinopyroxene, 3.7%, MORB, 14.0%, Q,
0.085% and CRUST, 0.555%.
Concentrations
of
certain
key
elements
are
sodium,
2994
ppm,
potassium,
205
ppm,