Geoscience Reference
In-Depth Information
chromium, cobalt, nickel, osmium and iridium,
some of the so-called compatible elements. The
continental crust is an important reservoir of
potassium, rubidium, barium, lanthanum, ura-
nium and thorium, some of the classical LIL ele-
ments. Thus, each of these components plays an
essential role in determining the overall chem-
istry of the primitive mantle.
It is conventional to adopt a single hypothet-
ical mix -- lherzolite or harzburgite plus basalt --
as the dominant silicate portion of the mantle;
this has been called
pyrolite
,for
pyroxene--olivine
rock
. An orthopyroxene-rich component (OPX) is
alsopresentinthemantleandisrequiredifsuch
major-element ratios as Mg/Si and Al/Ca ratios of
the Earth are to be chondritic. Clinopyroxenites,
rather than fertile peridotites, may be impor-
tant source rocks for basalts. Some peridotites
appear to have been enriched (metasomatized) by
a kimberlite-like (Q) component. Seawater is an
important repository of Cl, I and Br. The atmo-
sphere may contain most of the heavier rare
gases. Mixtures of the above components, plus
continental crust, can be expected to give a first
approximation to the composition of primitive
mantle.
There may also be inaccessible reservoirs
that do not provide samples for us to measure.
The so-called
missing element and isotope
paradoxes in geochemical box-models
suggest that some material is hidden away, prob-
ably in deep dense layers that formed during
the accretion of the Earth. Ratios such as Ca/Al,
Mg/Si, U/Pb and U/Nb and some isotope ratios
imply that there is hidden or inaccessible mate-
rial. The most obvious missing elements are
iron and other siderophiles, such as Os and
Ir. These are in the core. The missing silicon
is probably in the perovskite-rich lower mantle.
Other missing elements are S and C and other
volatiles that left the Earth entirely or were
never incorporated into it. There are numerous
paradoxes associated with U and Th and their
products -- heat, Pb-isotopes, He-isotopes and
Ne-isotopes. The obvious implication is that we
are missing something; the mantle may be
chemically stratified and we are sampling only
the outer reaches, or we are ignoring certain
components such as fluid-filled or melt inclu-
2.5
2.0
1.5
1.0
ALKALI
BASALT
KIMBERLITE
CONTINENTAL
CRUST
2.0
CONTINENTAL
THOLEIITE
1.5
1.0
10
×
7
×
0.5
MORB
0
K
Rb
Sr
Y
Zr
Nb Ba
La
Nd Sm Yb
Hf
Th
U
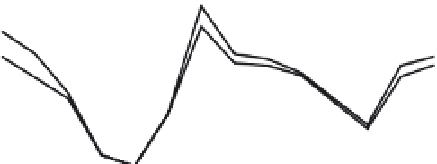
Fig. 13.1
Trace-element concentrations in the continental
crust (dots), continental basalts and midocean-ridge basalts
(MORB), normalized to average mantle compositions derived
from a chondritic model. Note the complementary
relationship between depleted basalts (MORB) and the other
materials. MORB and continental tholeiites are approximately
symmetric about a composition of 7
×
C1. This suggests that
about 14% of the Earth may be basalt. For other estimates,
see text.
sions, carbonatites and exotic minerals such as
rutile, osmiridium etc. It is possible that cer-
tain rock types such as lower-crustal cumulates,
carbonatites, recycled material and island-arc
basalts are not added into the mix in appropriate
quantities.
Figure 13.1 shows representative composi-
tions of kimberlite, crust, MORB and ultramafic
rock. For many refractory elements kimberlite
and crust have a similar enrichment pattern.
However, the volatile/refractory ratios are quite
different, as are ratios involving strontium,
hafnium, titanium, lithium, yttrium, ytterbium
and lutetium. Kimberlite and MORB patterns are
nearly mirror images for the refractory elements,
but this is only approximately true for MORB
and crust, especially for the HREE, and the small-
ion--high-charge elements. MORB and kimberlite
also represent extremes in their strontium and
neodymium isotope compositions.
When LIL-rich materials (KIMB, lamproites)
are mixed with a depleted magma (MORB), the
resulting blend can have apparently paradoxical
geochemical properties. For example, the hybrid