Geoscience Reference
In-Depth Information
in H
+
ions (so the pH) and in HCO
3
-
and CO
3
2-
ions, which contribute
to alkalinity, are interdependent
via
the equilibriums of dissolved
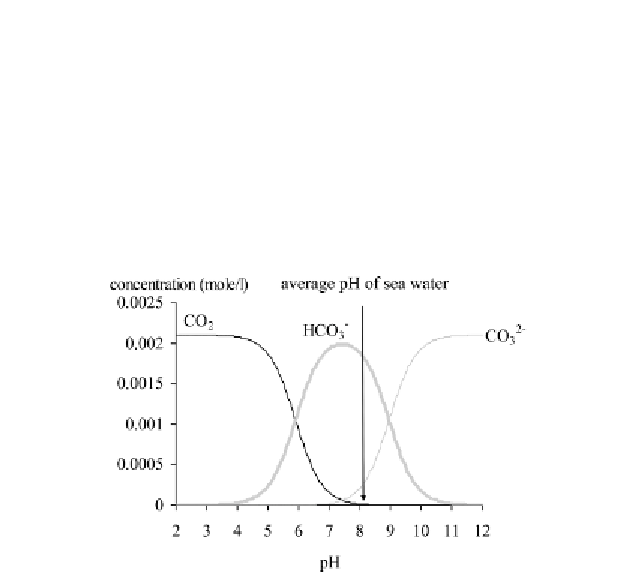
inorganic carbon, as shown in Figure 1.4. If the ocean becomes more
acidic (a drop in pH), the levels of CO
2
and HCO
3
-
increase while that
of CO
3
2-
diminishes. The consequences are an exportation of CO
2
into
the atmosphere, so an amplified greenhouse effect and conditions
favorable to the dissolving of solid carbonate. These conditions are
detrimental to the existence of some organisms that construct shells or
skeletons. The consequences are inversed if the pH increases.
Figure 1.4.
Relative distribution of three chemical forms of dissolved inorganic
carbon as a function of the pH in the average current conditions of seawater on the
Earth's surface (total concentration of inorganic dissolved carbon or DIC =
2.1 mmole.kg
-1
, salinity = 35 g of salts kg
-1
of seawater, temperature = 25°C) (from
[BER 08])
C
OMMENTARY ON
F
IGURE
1.4.- Because of the general composition
of dissolved compounds in seawater and with respect to electric
neutrality, the current average pH of seawater is adjusted to around
8.1 to 8.2.
Evidently, periodic variations in the concentration of borate ion
B(OH)
4
-
also play an important role in this adjustment and therefore
have an effect on the fluxes of CO
2
.
To summarize, the tectonic activity (either on land or on the ocean
floor), with the ocean as chemical mediator, is the primary cause of