Biology Reference
In-Depth Information
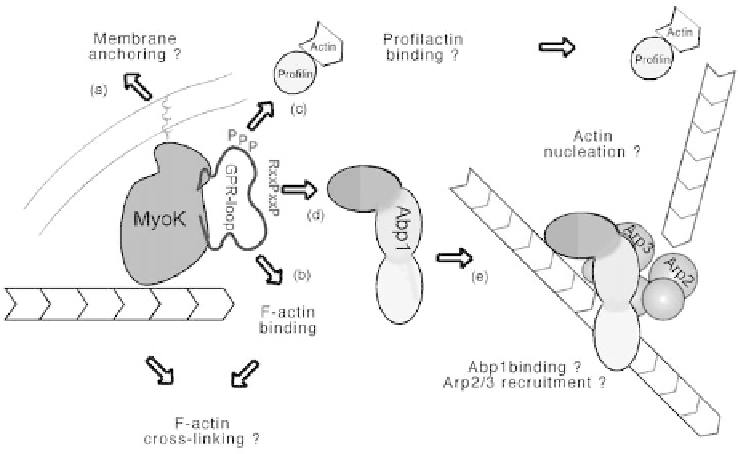
Figure 3.5 A working model of MyoK molecular functions. Results from the
investigation of MyoK function in vivo and in vitro (Schwarz et al., 2000) combined with
our most recent unpublished experiments allow us to propose the following working
hypothesis. First, MyoK is the first myosin carrying a C-terminal CAAX prenylation motif
and preliminary experiments indicate that the last 55 residues of MyoK mediate
prenylation and membrane attachment of a GFP fusion protein (a). Secondly, the GPR-
loop appears able to bind not only F-actin (b), but also the profilin-actin complex (c).
Thirdly, we identified the D. discoideum homologue of the yeast and mammal Abp1/
SH3P7r2 protein as a binding partner for the SH3 binding motif (d). Therefore, we propose
that MyoK, via its membrane anchor and anity for F-actin, could position (and activate)
the Arp2/3 complex (e) in close proximity of the plasma membrane. If MyoK motor
domain gets activated by phosphorylation of its TEDS site, it could maintain contact with
the barbed end of F-actin and participate in elevating the local concentration of the
profilin-actin complex, the 'fuel' for F-actin elongation
Conclusions and outlook
The C-terminal acidic domain that mediates the direct association of yeast
myosin I with the Arp2/3 complex is missing in the D. discoideum and
Acanthamoeba myosins I, but the connection to the Arp2/3 complex has been
maintained through the adaptor protein CARMIL. Homologues of CARMIL
exist in Caenorhabditis elegans (Jung et al., 2001), Drosophila and other higher
eukaryotes including mammals, providing an important clue that the long-
tailed class I myosins present in animals may be similarly linked to the Arp2/3
complex via their SH3 domain. Importantly, the example set by D. discoideum
MyoK of an alternative mode for the indirect recruitment of the Arp2/3