Biology Reference
In-Depth Information
et al., 1997; Titus, 1999; Tuxworth et al., 2001). Another striking aspect of the
dynamic localization of DdM7 is the gross similarity to that of the PI(3,4)P
2
/
PI(3,4,5)P
3
binding protein CRAC (cytosolic regulator of adenylyl cyclase),
which in addition to being recruited to the sites of chemoattractant binding is
also found in regions of dynamic actin polymerization such as phagocytic cups
and macropinocytic crowns (Parent et al., 1998), as is DdM7. It is possible
that DdM7 is recruited to the plasma membrane through localized production
of PI(3,4)P
2
and/or PI(3,4,5)P
3
that would occur immediately upon the
interaction of a receptor and its substrate. Once DdM7 is recruited to the
membrane it might then cooperatively interact with membrane-resident TalA
to organize adhesion receptors into the necessary high avidity complex
(Figure 2.2).
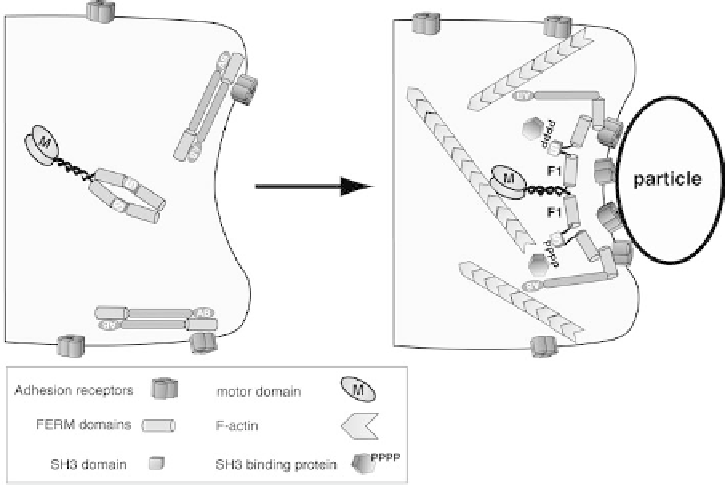
Figure 2.2 Speculative model of the shared contributions of Dictyostelium M7 and TalA
to adhesion. Shown is a region of a Dictyostelium cell prior to (left) and after (right)
binding a particle. Prior to engaging a particle or surface, cell surface adhesion receptors
are distributed along the surface of the cell, perhaps in association with talin (dimer of
elongated rods). A DdM7 dimer may be soluble in the cytoplasm. Initial contact with a
particle may trigger release of PIP
2
(not depicted) and result in recruitment of DdM7 to the
talin-receptor complex. Conformational changes in both DdM7 and TalA (only one TalA
molecule per dimer is shown for clarity) results in the assembly of a high avidity adhesion
receptor/TalA/DdM7 complex that then signals to the actin cytoskeleton, perhaps via
exposure of the DdM7 SH3 domain that would, in turn, recruit proteins that stimulate
localized actin polymerization at the plasma membrane and would drive an extension along
the surface