Biology Reference
In-Depth Information
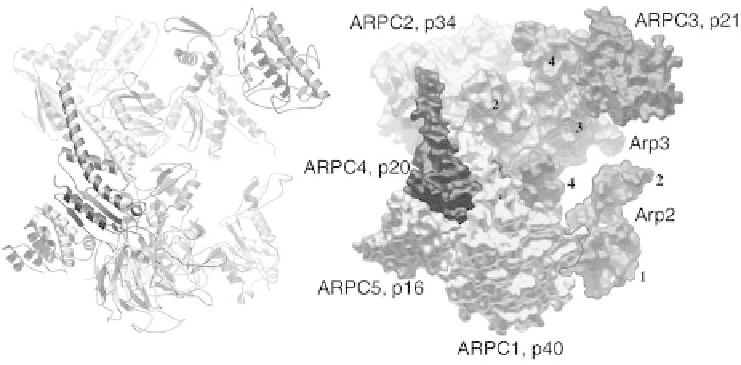
Figure 1.2 Crystal structure of inactive bovine Arp2/3 complex, illustrating the
arrangement of the seven subunits in ribbon and space filling models. Separation of the
Arps is postulated to account for the inactivity of the complex. Nucleation-promoting
factors are postulated to stabilize a more compact conformation with the Arps arranged
like adjacent subunits in an actin filament. (Modified from Robinson et al., 2001)
Robinson et al., 2001; C. Beltzner and T.D. Pollard, in press, 2004). The actin
related proteins Arp2 and Arp3 are folded exactly like actin, but with amino
acid substitutions and longer surface loops that participate in interactions
with the other subunits. ARPC1 (also called p40) is a b-propeller protein with
seven blades similar to a trimeric G-protein b subunit. A novel loop inserted
between blades 6 and 7 is postulated to interact with actin filaments at
branches. A dimer of two similar subunits (ARPC2 and ARPC4) holds the
complex together through extensive interactions of most of the other subunits.
ARPC3 and ARPC5 are a-helical subunits on the periphery. The conforma-
tion in the crystal is thought to be inactive, since physical separation of the
two Arps prevents them from initiating a new actin filament.
The ground state of the system
In the absence of positive stimuli, physiological concentrations of these
essential proteins will assemble a static gel. Roughly half of the actin will
assemble into filaments and the remainder will be bound to profilin (and
thymosin-b4 in vertebrates). Even pure actin filaments are quite stable under
physiological conditions in ATP. Owing to a small difference in the critical
concentrations for elongation at the two ends (Pollard, 1986), actin subunits
flux slowly onto the barbed end and off the pointed end, but the rate is less