Biology Reference
In-Depth Information
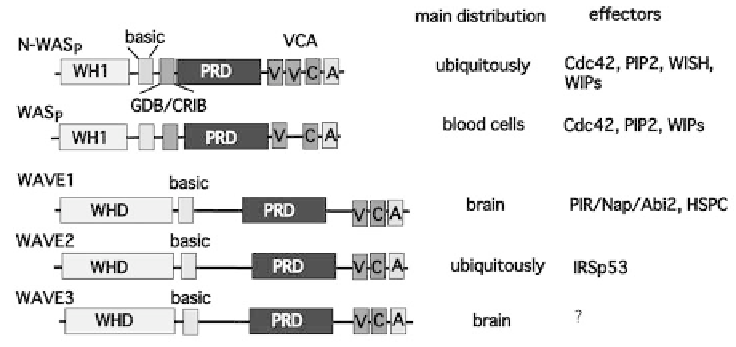
Figure 9.1 WASp and WAVE family proteins. The WASp family comprises N-WASp and
WASp, and the WAVE family comprises, WAVE 1, 2, and 3. These proteins all have VCA
region at the C-terminus through which these proteins activate the Arp2/3 complex, leading
to actin nucleation and polymerization
multifunctional domains including a WH1/EVH1 (WASp homology/Ena
VASP homology) domain, IQ motif, basic region, GBD/CRIB motif, proline-
rich region, verprolin-homology region (V), cofilin-like region (C), and acidic
region (A) (Figure 9.1). Available data suggest that WASp and N-WASp are
downstream targets of Cdc42, leading to filopodium formation (Miki et al.,
1996; Symons et al., 1996). WAVE1 was identified as a novel protein with a V
domain, following the identification of WAVE2 and WAVE3 (Miki et al.,
1998; Suetsugu et al., 1999). At the same time, a Dictyostelium homologue of
WAVE was identified and named Scar (Bear et al., 1998). WAVE1, 2 and 3 are
highly homologous to each other, and all contain a WAVE/Scar homology
domain (WHD) at the N-terminus, a basic region, a proline-rich region, and a
VCA region at C-terminus (Figure 9.1). Therefore, the C-terminal regions of
WAVEs are highly homologous to that of N-WASp. However, WAVEs do
not have a Cdc42-binding site or GBD/CRIB motif, suggesting that WAVEs
are regulated differently from WASps.
WASp and WAVE activate the Arp2/3 complex through the
VCA region
In resting cells, the barbed ends of actin filaments are protected by capping
proteins such as gelsolin superfamily proteins and capping protein, CapZ, to
prevent spontaneous, unregulated actin polymerization. Therefore, to induce
rapid actin polymerization at
the leading edge in response to stimuli,