Biology Reference
In-Depth Information
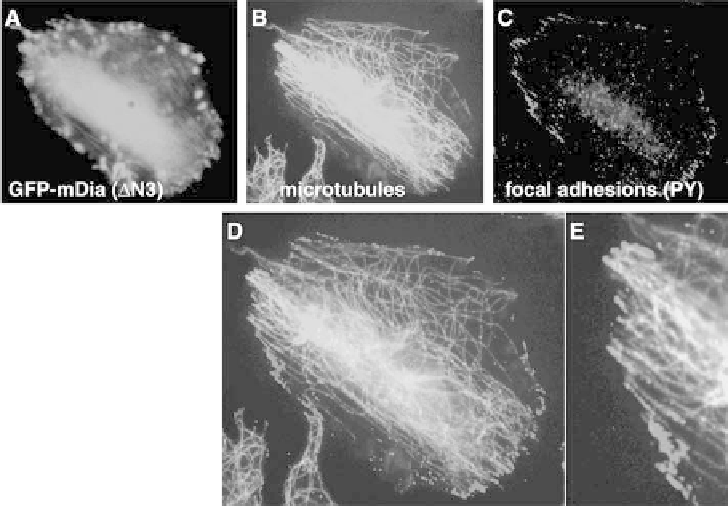
Figure 5.5 Microtubule ends at the periphery of active mDia1-expressing cells co-localize
with focal adhesions. CHO-K1 cells expressing mDia1 DN3-GFP (A) have a bipolar shape
with microtubules aligned along the axis (B). Many of the microtubule ends at the
periphery of the cell overlap with focal adhesions (visualized by anti-phosphotyrosine
antibody staining) (C). Frames (D) and (E) represent the merged microtubule and focal
adhesion images; E shows a part of D at higher magnification. (A colour reproduction of
this figure can be found in the colour plate section)
relevant, since their primary role in the regulation of microtubule dynamics
and targeting to the cell cortex both in yeast and in higher eukaryotic cells is
becoming increasingly clear (Gundersen, 2002). This group includes the
evolutionarily conserved proteins EB1, CLIP-170 and LIS1 and their
respective partners APC, CLASPs and the dynein-dynactin complex
(McNally, 2001; Schuyler and Pellman, 2001). There are certainly additional
links between the members of this group: LIS1 can bind CLIP-170 (Coquelle
et al., 2002), while EB1 binds p150Glued, an essential component of dynactin
(Askham et al., 2002), etc. Effects of several proteins from this group on
microtubule dynamics in vitro and in vivo are well documented (Komarova et
al., 2002a; Tirnauer et al., 2002). Xenopus XMAP215 and the kinesin family
microtubule-depolymerizing protein XKCM1 also regulate dynamics at the
microtubule ends (Kinoshita et al., 2001). Relationships between these
proteins and the proteins of the EB1/CLIP-170/LIS1 group are not yet
clear. While the microtubule tip-binding proteins are (by definition) localized
to the microtubule plus ends, it is interesting that many (if not all) members of