Chemistry Reference
In-Depth Information
Table 1.1
(continued)
Test
a
Application
Biothreat detection
Anthrax, plague, tularaemia, ricin, botulinum toxin, Staphylococcal
enterotoxin B, orthopox, Brucella, abrin, biological warfare
simulants, nerve (G&V series), Category A-C biothreat agents, and
blister (HD) agents, acids, bases, aldehydes and oxidisers [
65
-
67
]
a
Require minimal sample preparation
b
Urine collection and detection are often integrated in one cup
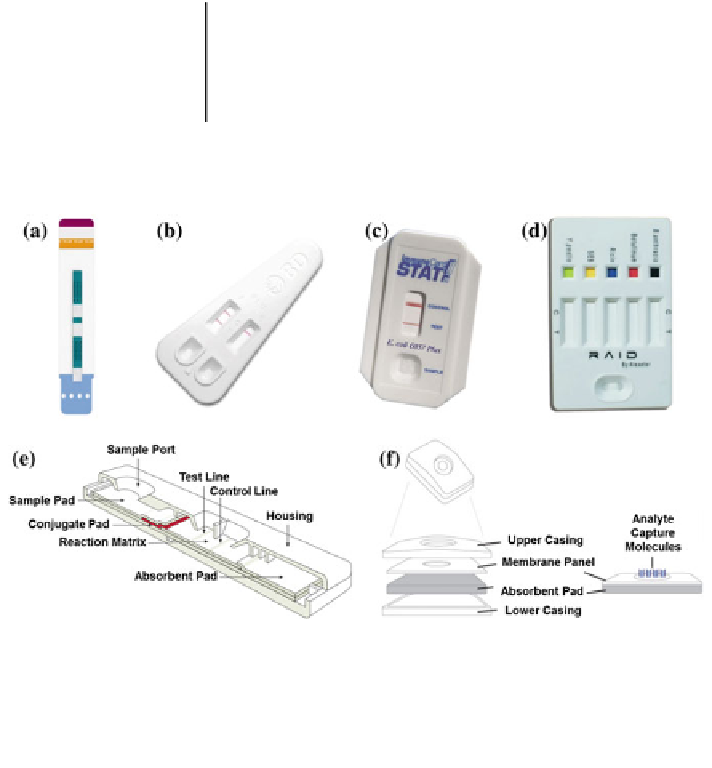
ow point-of-care assays in the market and their cassette formats.
a
Determine
™
TB LAM Ag test.
©
(Alere),
b
Directigen
™
EZ Flu A + B (Beckton Dickinson),
c
ImmunoCard
STAT!
®
E. coli O157 Plus (Meridian Bioscience),
d
A multiplex lateral-
Fig. 1.1
Lateral-
fl
ow assay. RAID
™
5 for
biological threat detection (Alexeter Technologies),
e
Schematic of the lateral-
fl
fl
ow assay, and
f
flow-through assay. Adapted from Ref. [
69
] with permission from The Royal Society of
Chemistry
fl
Typical approaches include the removal of contaminants from the samples to improve
selectivity and sensitivity, and decrease the turnaround time. Additionally, the sample
might require further processing to improve the signal-to-noise ratio. Sample prepa-
ration suffers from inhomogeneity, interfering agents, inhibitors, and it requires
increasing the viscosity of samples such as whole blood and food samples. Increasing
the sensitivity requires tedious sample preparation steps for low concentrations of
target molecules or cells. The ideal sample preparation step should be cost-effective
and is potentially executed in a single step. The desired outcome of the sample
preparation is concentrating the target analyte(s) and reducing the background noise
due to matrix interferences. Lateral-
ow tests are low-cost, lightweight, portable, but
the growing demand for higher sensitivity is challenging its current format [
68
]. For
fl