Chemistry Reference
In-Depth Information
5.6 Kinetic Theory for Hydrogel Swelling
The capability for the glucose binding to occur is associated with the amount of cis
diols available within the polymer matrix and the concentration of glucose in the
sample measured. A change in the number of molecules bound
ʷ
(t) is:
d
dt
g
ðÞ/b
t
ð
N
g
ðÞ
t
Þ ¼
c
1
bð
N
gð
t
ÞÞ
ð
5
2
Þ
:
where t is the measurement time,
is the concentration of glucose, N is the number
of cis diols (bound and not bound),
ʲ
(t) is the number of cis diols already bound
with glucose molecules, and c
1
is a constant. This expression implies that the
concentration
ʷ
is a constant, in other words, the amount of glucose molecules is in
excess than the amount of cis diols and therefore, it is assumed that the concen-
tration does not decrease. This relationship can be expressed as:
ʲ
c
2
e
c
1
b
t
g
ðÞ¼
t
N
ð
5
3
Þ
:
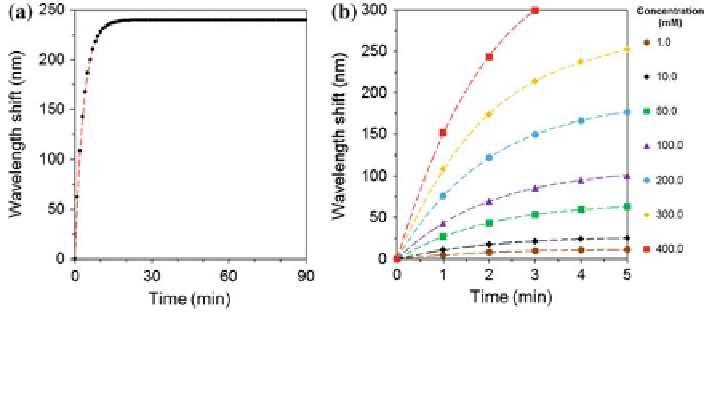
Figure
5.16
a shows a typical simulated readout from a holographic sensor. When
the Bragg peak measurement is taken from the poly(AAm-co-3-APB) matrix, the
binding process may have already started, in such a case, the initial measurement is
not t = 0 but an arbitrary t
i
. The difference of
ʷ
(t) from a
xed t
i
to the next
measurement time t can be expressed as:
c
2
e
c
1
b
t
i
e
c
1
b
t
t
i
ð
Þ
e
c
1
b
t
t
i
ð
Þ
D
g
ðÞ¼g
t
ðÞg
t
t
ðÞ¼
1
¼
c
3
ð
1
Þ
ð
5
4
Þ
:
Fig. 5.16 The systematic approach to extrapolate the readouts using kinetics of hydrogel swelling.
a A typical sensor response showing the correlation between time (min) and Bragg peak shift (nm).
As the time increases, the Bragg peak shift saturates after a time point. b The initial calibration
curves set for the measurements