Chemistry Reference
In-Depth Information
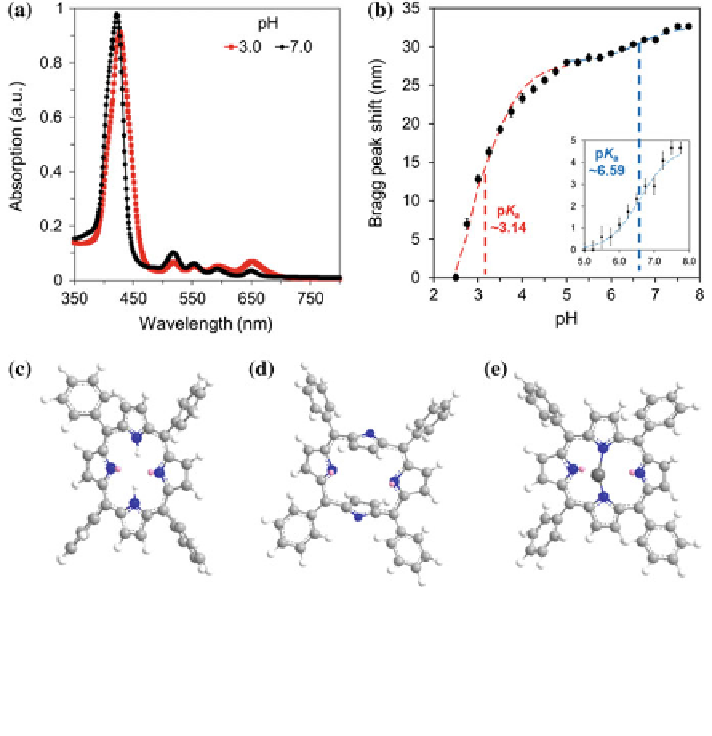
Fig. 4.4 Determination of the apparent pK
a
value and activation of TACPP cavity. a Ultraviolet-
visible spectra of pHEMA-co-TACPP matrix.As the pH was changed from 7.0 to 3.0, the
absorption peak shifted from 420 to 426 nm. b The apparent pK
a
values for the porphyrin-
crosslinked pHEMA matrix were calculated using the Henderson-Hasselbalch equation. TACPP
states in c neutral, d deprotonated, and e chelated with a divalent cation. Reproduced from Ref. [
1
]
with permission from The Royal Society of Chemistry
atoms are located below and above the macrocycle plane of the porphyrin
(Fig.
4.4
e) [
20
]. After the cavity of TACPP was deprotonated, the pHEMA-co-
TACPP matrix was saturated with mono/divalent ions (1.0 M) in buffer solutions.
In response to metal cations, the Bragg peak of the pHEMA-co-TACPP matrix blue
shifted (Fig.
4.5
a, b). The shrinkage in the matrix was due to (i) electrostatic
interactions, which decreased the Donnan osmotic pressure, and (ii) TACPP and
metal cation chelation that changed the conformation of the molecule. By rinsing
the system with DI water, the electrostatic interaction was eliminated, which
allowed the measurement of the relative blue Bragg shift due to the chelation
(Fig.
4.5
c). The blue Bragg shifts for Cu
2+
and Fe
2+
ions were 5.24 and 4.66 nm,
which were more pronounced than other mono/divalent cations. When the system
was rinsed with the carbonate buffer and pure DI water, the Bragg peak shifted back
to its original position (
523 nm). In the readouts, the absorption of Cu
2+
and Fe
2+
ions in the polymer matrix was negligible as compared to the Bragg peak shift.
*