Environmental Engineering Reference
In-Depth Information
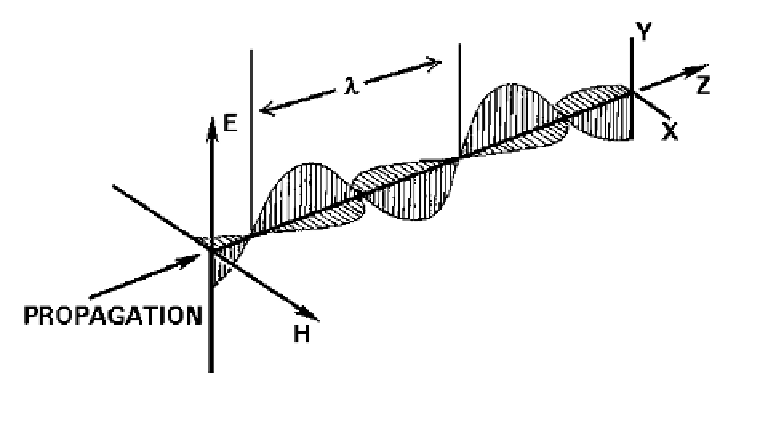
Figure 2.
The wavelength is the distance between two corresponding points at the same location from
cycle to cycle.
Light, like all electromagnetic radiation, appears to be a wave in some
phenomena, but behaves as if it were made up of infinitely small particles when we
observe other physical phenomena. At one time this was considered a great paradox.
Some scientists argued that light behaved like a wave and was clearly wave-like in
nature. Others argued that light really behaved like particles and pointed to some
phenomena that could only be explained if light were really particles, or photons
(quanta of light energy). However, with the development of the quantum theory of
radiation about a century ago, physicists began to become accustomed to this
“Wave-Particle Duality,” and simply accepted that light was both a wave and a particle!
Light demonstrates both phenomena. The relationship between frequency and photon
energy Q is:
Q
Ȟ
= h·Ȟ (1)
and since:
c = Ȝ·Ȟ
(2)
then:
QȞ = h·c/Ȝ (3)
where the constants are the speed of light = c = 3.00 x 10
8
meters per second; and
Planck's Constant = h = 6.63 x 10
-34
joule per second.
When one enters the world of radiation biophysics and the effects of
electromagnetic radiation upon biological systems, it is frequently useful to separate the
entire EM spectrum into ionizing and non-ionizing radiation. Ionizing radiation has
enough photon energy to create ion pairs; that is, to make an atom gain or lose
Search WWH ::

Custom Search