Biology Reference
In-Depth Information
1.1. Potassium
Channels
The largest and a highly diverse group of ion channels are constituted
by potassium-selective channels (K
+
channels). Out of this group
with about 70 gene loci in the mammalian genome, four distinct
K
+
channel types are important in vascular smooth muscles and
subsequently responsible for the development of SAH-induced
vasospasm. Voltage-dependent potassium channels (K
v
) form a
group with 12 subfamilies and about 40 genes. Their pore-forming
α
-subunit has a six transmembrane domain structure with a cyto-
plasmatic N- and C-terminus (reviewed in ref. (
6
)). Large conduc-
tance Ca
2+
-activated K
+
channels (BK
Ca
) also exhibit a six
transmembrane domain structure. However, they are activated by
raised intracellular Ca
2+
and membrane depolarization (reviewed in
ref. (
6
)). ATP-sensitive K
+
channels (K
ATP
) are highly diverse, small,
or large conductance channels constituted by four pore-forming
subunits of inwardly rectifying channels (Kir) and sulphonylurea
receptors (reviewed in ref. (
6
)).
Voltage-activated Ca
2+
channels are heterooligomeric protein com-
plexes characterized by their central pore-forming unit (
1.2. Calcium Channels
1-subunit;
Fig.
1
). Since discovery of voltage-activated Ca
2+
channels in the
early 1950s, ten different genes of the ion conducting subunit have
been described (Table
1
). Basically, these channels could be divided
in high voltage-activated and low voltage-activated calcium chan-
nels. High voltage-activated Ca
2+
channels require a high shift in
membrane voltage for activation (
7-9
) and could be divided into
two subgroups: The Ca
v
1 subfamily or L-type Ca
2+
channels with
their pore-forming subunits Ca
v
1.1 (
α
α
1S), Ca
v
1.2 (
α
1C), Ca
v
1.3
(
1F) activate at a membrane potential of about
−30 mV, exhibit a comparatively slow inactivation and high chan-
nel conductance and are inhibited by typical L-type Ca
2+
channel
blockers (Table
1
). The second group of high voltage-activated
α
1D), and Ca
v
1.4 (
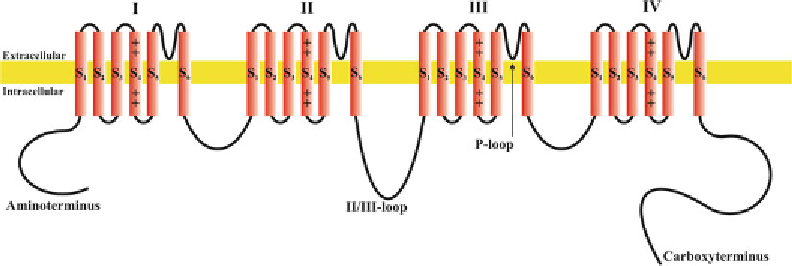
α
Fig. 1. Structure of voltage-gated ion channels. As example for a structure of a voltage-gated ion channel, pore-forming
α
-subunit of voltage-gated Ca
2+
channels is shown. The
α
1-subunit contains of Ca
2+
channels contain four homologous
domains (I-IV) with each six membrane-spanning
α
-helices (S1-S6). The carboxy- and aminoterminus as well as the loops
connecting the domains are situated on the cytoplamatic site of the plasma membrane.
Search WWH ::

Custom Search