Biology Reference
In-Depth Information
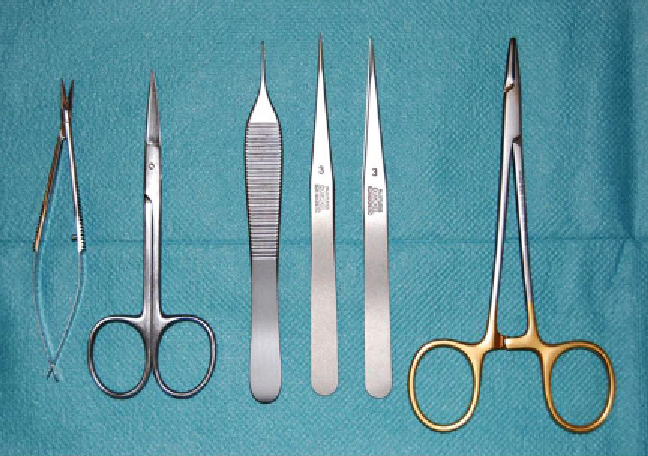
Fig. 3. Surgical instruments required for tissue preparation.
Left
to
right
: microscissors, surgical scissors, anatomical and
microsurgical forceps, and needle driver.
In order to measure cortical tissue perfusion after experimental
SAH (the method of experimental SAH in mice is detailed in a
separate chapter), the LASCA measurement may be repeatedly
performed and as early as immediately after the SAH procedure.
However, it is important to note, that frequent opening and sutur-
ing of the scalp leads to the formation of highly vascularized scar
tissue, which needs to be considered during surgical preparation.
2.3. Procedure
In order to avoid CBF alterations by volatile anesthetics and to
facilitate handling and repositioning of the animal, anesthesia can
easily be performed with an intraperitoneal injection of 70 mg/kg
ketamine and 16 mg/kg xylazine. The estimated time of suffi cient
anesthesia with this dosage is up to 45 min. To ensure stable base-
line fl ux values, a time period of 15-20 min after induction of anes-
thesia is recommended before starting the LASCA measurement.
2.3.1. Anesthesia
In order to guarantee suffi cient time for LASCA measurements,
the following procedure should require no more than 7-10 min:
The scalp is shaved and disinfected with alcohol before the
mouse is placed on a portable pad in prone position (Fig.
4a
).
The limbs can be optionally secured to avoid unintentional shift-
ing/movement of the animal. Next, the fronto-parieto-occipital
scull is bilaterally exposed through a linear 2 cm, midline scalp
incision. To prevent drying of the scull (which changes optical
density of the bone), the surgical fi eld is irrigated with sterile saline.
The scalp is gently retracted laterally and retraction is sustained by
2.3.2. Scalp Exposition
(Optionally with Surgical
Microscope)
Search WWH ::

Custom Search