Biology Reference
In-Depth Information
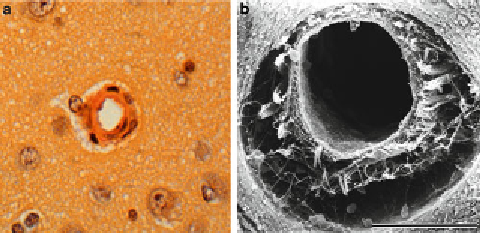
Fig. 2. (
a
) Light microscopy of canine intraparenchymal arteriole, (
b
) scanning electron
microscopy of canine intraparenchymal arteriole.
whereas in scanning microscopy, a specimen of the depth to be
aimed for is taken out, and its surface is observed (Fig.
2b
).
2. Observation of microvascular architecture
(a) Casting method (Fig.
3a-c
) (
4, 5
).
After perfusion-fixation, polyester resin (Mercox) is
injected through the same catheter at the same pressure as
perfusion-fi xation. In order to maintain the same pressure,
the catheter should also be connected to a pressure meter
with a 3-way stopcock to confi rm the injection pressure.
The polyester resin recently released is formulated with an
extremely low viscosity and does not require dilution with
methyl methacrylate to lower the viscosity. The volume of
polyester resin to be injected depends on the animal used.
The time of polymerization of polyester resin depends
upon the mixture ratio of resin and catalyst, which should
be adjusted according to the experiment. After the polym-
erization is completed, the brain is removed. During the
removal of the brain, retraction of the brain should be
minimized to avoid destroying the cast.
The tissue block is kept in 20% NaOH. The time
required for corroding away the brain tissue is from several
weeks to several months. If the tissue plaques remain on
the plastic cast, they are removed by ultrasonic cleaning
for 1 day. The cleaned cast is freeze-dried for 1 day to pre-
serve its fi ne structure. The cast is mounted, sputter-coated
with gold in an Ioncoater, and examined using scanning
electron microscopy.
(b) Carbon black infusion (Fig.
4a
) (
6
)
After perfusion-fi xation, mixed solution with 5-20% gela-
tin and 10-40% India ink is injected via the same catheter
used in perfusion-fi xation. After injection, the animal is
Search WWH ::

Custom Search