Biology Reference
In-Depth Information
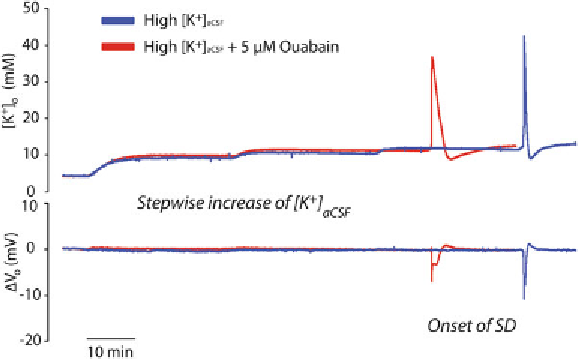
Fig. 1. Exemplary recordings showing SD in a mouse brain slice after stepwise increase of
[K
+
] in the bathing medium with (
red
) and without (
blue
) ouabain (5
μ
M). Recordings have
been obtained employing K
+
selective/reference electrodes located in layer II/III of the
somatosensory cortex. The specifi c Na,K-ATPase inhibitor ouabain reduces the SD threshold
and prolongs the accompanied extracellular K
+
surge as well as the slow fi eld potential shift.
150 to 60 mM, [Cl
−
]
o
from 140 to 90 mM, and [Ca
2+
]
o
from 1.3
to 0.1 mM; SD is accompanied by transient alkalinization and fol-
lowed by sustained acidifi cation (
1, 2, 24
). The low propagation
rate (2-8 mm/min) of SD is usually calculated from the distance
between the electrodes and the 50% maximum times of the nega-
tive potential shift recorded by the electrodes or by use of imaging
techniques.
To assess extracellular electrophysiological characteristics of
SD, we employ standard ion-selective microelectrodes to measure
simultaneously changes in fi eld potential and [K
+
]
o
(Fig.
1
). Ion-
selective microelectrodes are prepared using double-barreled theta
glass (
25, 26
). One barrel is fi lled with 154 mM NaCl and serves
as the reference. The other barrel is silanized (5% trimethyl-
1-chlorosilane in 95% CCl
4
) and fi lled with a potassium ionophore
cocktail (Fluka A60031, 60398, or Corning 477317). To calculate
extracellular potassium concentrations from the recorded potential
values, a modifi ed Nernst equation is employed (
27
): log[Ion]
1
=
E
M
× (
s
×
v
)
−1
+ log[Ion]
o
, with
E
M
, recorded potential;
s
, electrode
slope obtained at calibration;
v
, valence of the specifi c ion; [Ion]
o
,
extracellular ion concentration at rest; and [Ion]
1
, ion concentra-
tion during activation. Using a similar approach by employing dif-
ferent ion exchanger resins, it is possible to assess extracellular Na
+
,
Cl
−
, Ca
2+
, and H
+
(pH) concentrations. Measurement of [Mg
2+
]
o
is
currently not possible using ion-selective microelectrodes since
available ion exchangers show a high selectivity coeffi cient for
Search WWH ::

Custom Search