Biology Reference
In-Depth Information
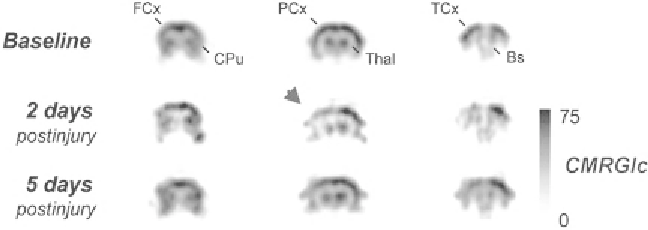
Fig. 3.
18
F-FDG microPET after experimental TBI. Preinjury glucose metabolism
( top row )
compared to metabolism 2 days
( middle )
and 5 days
( bottom )
after lateral fl uid percussion in rats. Both time points show reduced metabolism in the
hemisphere ipsilateral to brain injury (
arrowhead
). FCx = frontal cortex, CPu = caudate putamen, PCx = parietal cortex,
Thal = thalamus, TCx = temporal cortex, Bs = brain stem, CMRGlc = Cerebral metabolic rate of glucose. Adapted with per-
mission from Macmillan Publishers Ltd: J Cereb Blood Flow Metab 20:1492-1501, copyright 2000.
(the time between
18
F-FDG injection and PET scanning, usually
around 30-60 min), then the PET images will refl ect stimulus-
induced neuronal activation during that time window. This novel
application of micro-PET has not yet been utilized in neurotrauma
research. A notable recent technical development for micro-PET
was the optimization of
18
F-FDG neuroimaging after intraperito-
neal injection of tracer (
22
). This delivery procedure is easier and
faster than intravenous injection and should improve the feasibility
of future preclinical metabolic studies with micro-PET.
The continued development of novel tracer molecules is
expanding the potential research applications of PET neuroimag-
ing. New applications of micro-PET include noninvasive analyses
of gene expression, tracking neuroinfl ammatory changes, evaluat-
ing neurotransmitter receptor distribution, and in vivo monitoring
of transplanted stem cells. So far however, few investigators have
applied these newer micro-PET approaches to neurotrauma
research. In one recent study, Zhang and colleagues engineered
stem cells to express a specifi c dopamine receptor subtype, trans-
planted the cells into brain injured rats, and used a radioligand for
that receptor to track the distribution of the transplanted cells (
23
).
This approach may offer advantages over imaging grafted stem
cells with MRI, since presumably only living, viable stem cells can
express the receptor that binds the PET tracer. By contrast, MRI
has been criticized for being unable to differentiate between living
and dead labeled cells following transplant.
The recent development of PET tracers for imaging amyloid
protein accumulations in Alzheimer's disease (reviewed in ref.
(
24
)) raises the intriguing possibility that a similar tracer could be
designed to image amyloid precursor protein (APP) in vivo after
TBI. APP accumulation is a hallmark of diffuse axonal injury, a
component of TBI pathology that is often not detectable with
conventional neuroimaging. To date, defi nitive detection of axonal
APP accumulation has only been achieved with postmortem
Search WWH ::

Custom Search