Biology Reference
In-Depth Information
membrane around the electrode. The resting membrane poten-
tial of the cell should be at ~−60 mV without any current injec-
tion. The series resistance and the capacitance should be
readjusted before collecting the electrophysiological data.
7. Intracellular staining:
For intracellular staining, the recording electrode is fi lled with
3-5% neurobiotin (Vector Lab) in 2 M potassium acetate. At
the end of the recording, depolarizing current pulses (2 Hz,
300-ms duration, 0.5-1.0 nA) are delivered for 10-30 min to
inject the neurobiotin into the cell. In an experiment, more
than one neuron could be recorded and stained. In order to
match the electrophysiological data with the morphology
of the neuron, it is essential to unequivocally identify the
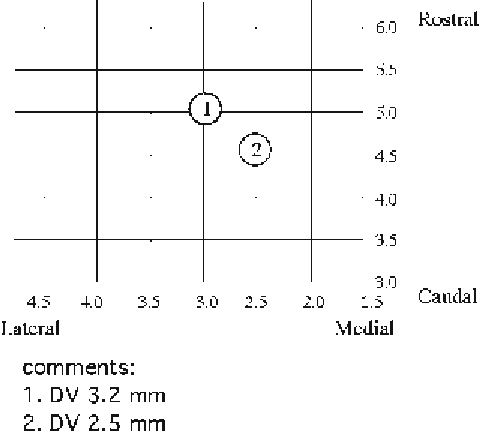
neurons after histological examination. We develop a chart to
record the 3D parameters of the location of the recorded neu-
rons (Fig.
4
). In the chart, the numbers of the horizontal
axis are the measurement from the midline of the skull and the
numbers of the vertical axis are from the interaural line (
9
).
First, write down the mediolateral and rostrocaudal parameters
when placing the electrode into the recording site. After a success-
ful intracellular staining, a note should be made for the depth
of the neuron. The next recorded electrode should be placed
at least 0.5 mm apart from the previous one. Based on these
3D locations, it is relatively easy to identify the neurons for
which the intracellular recording and staining are performed.
Fig. 4. Chart for the location of the recorded neurons.
Circles 1
and
2
are examples of recorded
neurons with the depth from the cortical surface in comments.
DV
dorsal-ventral.
Search WWH ::

Custom Search