Biology Reference
In-Depth Information
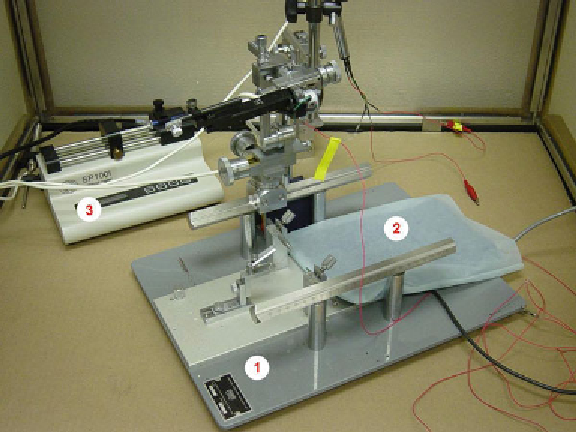
Fig. 2. Representative of an atereotaxic frame (Model SR-6 M, Narashige, Japan, Fig. 2-1),
a homeothermic blanket control unit and heating pad from Harvard Apparatus (Fig. 2-2),
and an automatic syringe pump from Harvard Apparatus (Fig. 2-3).
2.1.5. Data Acquisition and
Analysis Software
For EP experiments, we use pClampex 9.2 for data acquisition and
pClampfi t 9.2 for data analysis for our PC. For single-unit recording,
we use software package from Sky-A4 Bioelectric signal processing
system Mathlib (Fudan University; Shanghai, China).
2.2. Other Equipment
We use vibration isolation tables (Fig.
1b
-6, c-8) and a Faraday
cage (Fig.
1b
-5, c-7) from TMC for both EP and single-unit
recording.
2.2.1. Vibration Isolated
Table and Faraday Cage
2.2.2. Electrode Puller
We use PP-830 vertical electrode puller (Narashige, Japan).
We use Model SR-6M (Narashige, Japan, Fig.
2
-1).
2.2.3. Stereotaxic Frame
We use Narashige P-5N Motion controller (Fig.
1c
-4).
2.2.4. Electrode
Manipulator
Our homeothermic blanket control unit and heating pad are from
Harvard Apparatus (Fig.
2
-2).
2.2.5. Heater
2.3. Anesthesia
EEG
. (Rodents): 87 mg/kg ketamine plus 13 mg/kg xylazine (i.p.)
EP
. (Cat):
-chloralose 60 mg/kg (i.v.)
Single-unit recording
. (Rodents): Chloral hydrate (400 mg/kg, i.p.)
α
Search WWH ::

Custom Search