Environmental Engineering Reference
In-Depth Information
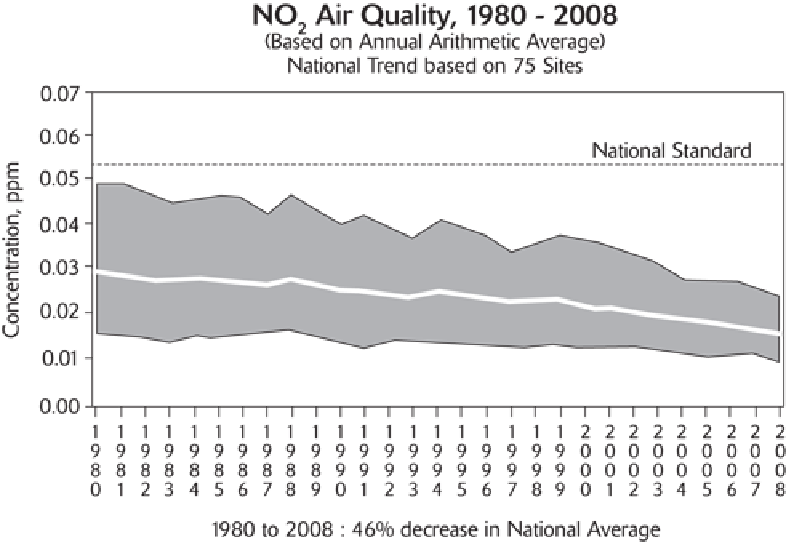
Source: Environmental Protection Agency
Sulfur dioxide (SO
2
)
is formed when sulfur is released from burning coal and oil, and then reacts with oxygen
in the atmosphere to form sulfur dioxide. The majority of atmospheric SO
2
is due to emissions from coal-fired
power plants. SO
2
can react with water vapor to form sulfuric acid (H
2
SO
4
) and sulfate salts, which can cause
acid rain. Acid rain can harm vegetation and speed the deterioration of structures such as buildings and statues.
Also, SO
2
absorbs ultraviolet radiation in the atmosphere to form
industrial smog.
It also can produce
aero-
sols,
which are solid particles and droplets suspended in the atmosphere. Naturally, SO
2
can be released from
volcanic activity.
Control of SO
2
emissions is a major goal of the National Ambient Air Quality Standards established by the
EPA under the authority of the Clean Air Act. These standards regulate emissions and develop plans to reduce
and monitor pollutants. One successful mandate of the National Ambient Air Quality Standards required the
extraction of sulfur from coal prior to combustion. Through extensive efforts, sulfur dioxide in the atmosphere
has decreased, but it is by no means eradicated.