Environmental Engineering Reference
In-Depth Information
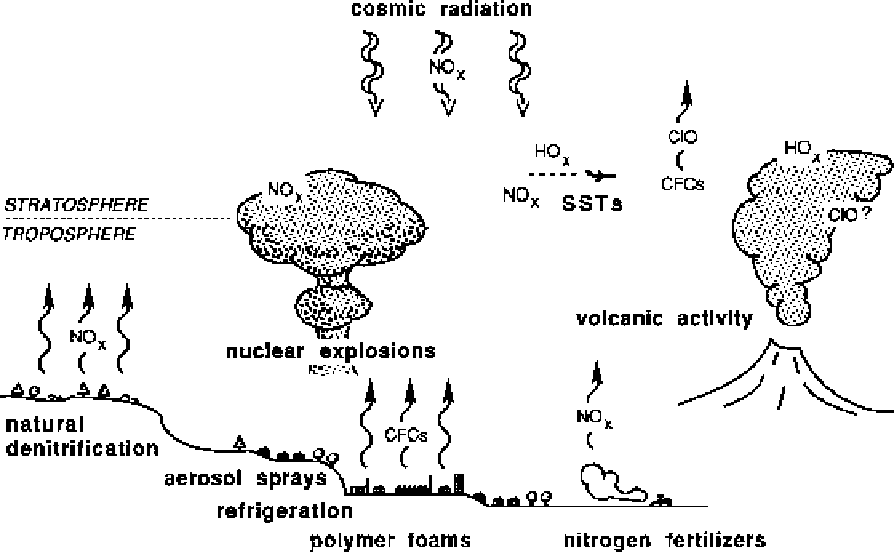
Figure 6.2
Diagrammatic representation of the sources of natural and anthropogenic ozone-destroyers
1972), and, although relatively low in total
volume, they affect ozone strongly, particularly
above 40km. Hammond and Maugh (1974) have
estimated that the HO
X
group, through its
catalytic properties, is responsible for about 11
per cent of the natural destruction of ozone in
the stratosphere (see Figure 6.3). The odd
hydrogens lose their catalytic capabilities when
they are converted to water vapour.
may also be produced in smaller quantities by the
action of cosmic rays on atmospheric gases
(Hammond and Maugh 1974). Major cosmic ray
activity in the past, associated with supernovas,
possibly produced sufficient NO
x
to cause a 90 per
cent reduction in the ozone concentration for
periods of as much as a century (Ruderman 1974).
The catalytic chain reaction created by NO is a
long one. Nitric oxide diffuses only slowly into the
lower stratosphere where it is converted into nitric
acid, and eventually falls out of the atmosphere in
rain. In contrast to the other oxides of nitrogen,
the presence of nitrogen dioxide (NO
2
) in the
lower stratosphere can be beneficial to the ozone
layer. It readily combines with chlorine monoxide
(ClO), one of the most efficient ozone destroyers,
to produce chlorine nitrate (ClONO
2
), a much less
reactive compound, thus providing some
protection for lower stratospheric ozone
(Brasseur and Granier 1992).
Nitrogen oxides
Nitrogen oxides (NO
x
) are very effective
destroyers of ozone (see Figure 6.4). Nitric oxide
(NO) is most important, being responsible for 50-
70 per cent of the natural destruction of
stratospheric ozone (Hammond and Maugh
1974). It is produced in the stratosphere by the
oxidation of nitrous oxide (N
2
O), which has been
formed at the earth's surface by the action of
denitrifying bacteria on nitrites and nitrates. It