Biomedical Engineering Reference
In-Depth Information
Many authors used FRET mechanism to produce singlet oxygen between a QD
and a photosensitizer covalently or not covalently coupled. The systems are com-
posed of CdSe, CdSe/CdS/ZnS, CdSe/ZnS and CdTe QDs as energy donors and
porphyrins, chlorins, phthalocyanines, inorganic complexes or organic dyes as
energy acceptors.
For example, Shi et al. (
2006
) proved that a water-soluble CdTe QD with
2-aminoethanethiol as surface stabilizer was not able to produce singlet oxygen by
itself but did after excitation of a
meso
-tetra(4-sulfonatophenyl)porphyrin dihydro-
chloride (Fig.
30
) bound to CdTe QD
via
electrostatic interaction. Jhonsi and
Renganathan (
2010
) investigated the photoinduced interaction of water soluble
thioglycolic acid capped CdTe Qds with porphyrins (Fig.
30
). The porphyrins were
found to be adsorbed in the surface of the QDs. Negatively charged porphyrins
were involved in the energy transfer mechanism whereas positively charges ones
involved electron transfer from QDs to porphyrin. The neutral porphyrin did not
have any interaction with QD.
Tsay et al. (
2007
) coated chlorin e6 (Fig.
31
) on the surface of CdSe/CdS/ZnS
QDs which provided
1
O
2
at 31% efficiency.
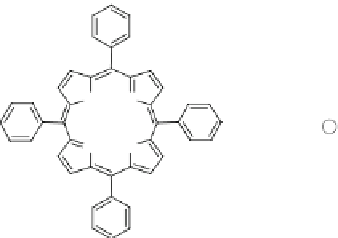
R
R = H
R = SO
3
R = COO
meso-tetraphenylporphyrin (TPP)
meso-tetrakis(4-sulphonatophenyl)porphyrin (TSPP)
NH
N
R
R
meso-tetrakis(4-carboxyphenyl)porphyrin (TCPP)
N
HN
R = CH
3
meso-tetra(4-N-methylpyridyl)porphyrin (TMPyP)
R
Fig. 30
Porphyrins used by Jhonsi and Renganathan
NH
N
N
HN
O
HO
O
O
OH
OH
Fig. 31
Chlorin e6



















Search WWH ::

Custom Search