Biomedical Engineering Reference
In-Depth Information
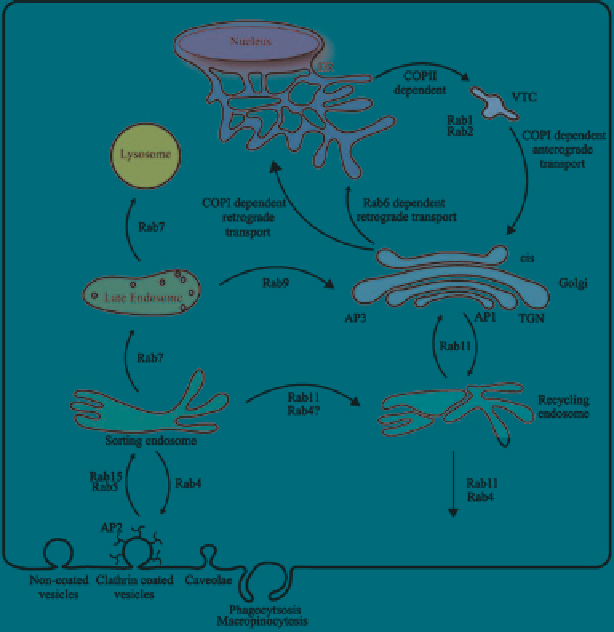
Fig. 4
A summary of membrane-protein-mediated trafficking (Reprinted from Watson et al.
(
2005
). Courtesy of Elsevier Publishing Group)
alter adenylyl cyclase activity. Other proteins, such as Shiga toxin, Pseudomonas
exotoxin and ricin toxin, utilize a similar route: the peptides are internalized via
caveolin or clathrin-mediated processes and trafficked to the ER, whereupon the
peptides exploit an unfolded-protein degradation/escape mechanism to enter the
cytosol and cause their respective cellular dysfunctions (Sandvig et al.
2010
).
Intracellular membrane trafficking mechanisms vary based on cargo. These dif-
ferent mechanisms allow for specific localization and different interactions en route
(Fig.
4
). Clathrin-mediated endocytosis traffics to endosomes where cargo can be
either recycled (Rab4), sorted for degradation (lysosomes) or delivered to the Golgi
(Rab 11 perinuclear sorting) (Rajendran et al.
2010
; Fantini et al.
2002
). To enter
cells, viruses and bacterial toxins utilize lipid rafts, which are enriched in choles-
terol and sphingolipids. Clustering of these raft components induces budding, and
these vesicles follow a pathway that bypasses early endosomes and traffics to the
Golgi (Fantini et al.
2002
).
Studies to specifically target nanoparticles to the ER are rare. Quantum dots (QD)
have been coupled to numerous ligands, such as Shiga toxin and ricin, to study
Search WWH ::

Custom Search