Biomedical Engineering Reference
In-Depth Information
ovaries, lungs, and adrenal glands. The fluorescence intensities decreased over
time in these organs, except for the liver. No urine elimination was observed
throughout 24 h.
Recently, the Prasad group used ORMOSIL nanoparticles, embedded with
HPPH (Fig.
13
) but covalently iodine-concentrated (Kim et al.
2009b
). These par-
ticles were prepared using (3-iodopropyl)-trimethoxysilane and vinyltriethoxysi-
lane. The properties of these nanoparticles were compared with noniodinated
nanoparticles (prepared from vinyltriethoxysilane) and it was shown that the intra-
particle external heavy-atoms significantly enhanced the efficiency of
1
O
2
genera-
tion and the
in vitro
PDT efficiency. Indeed, the quantity of
1
O
2
generated by the
iodinated nanoparticles was found to be increased by roughly 1.7 times over the
non iodinated nanoparticles. Cell viability assay was carried out for RIF-1 tumor
cells and nanoparticles with iodine were the most efficient for PDT applications.
Wang and collaborators entrapped methylene blue (Fig.
11
) in a phosphonate-
terminated silica matrix by controlled synchronous hydrolysis of tetraethoxysilane
and trihydroxyl silyl propyl methyl phosphonate in a water-in-oil microemulsion
(He et al.
2009
). Spherical nanoparticles were obtained with a mean diameter of
105 nm.
1
O
2
generation was evaluated using DPBF and estimated to be 0.049.
In
vitro
PDT efficiency was investigated on HeLa cells. Cell photocytotoxicity was
evaluated to 90% using 1 mg/mL methylene blue doped nanoparticles upon 30 min
light exposure (635 nm laser, 27.5 mW/cm
2
). Real-time
in vivo
imaging experi-
ments on BALB/c nude mice bearing MB-encapsulating nanoparticles were per-
formed. The methylene blue doped nanoparticles were injected intravenously in
nude mice for near-infrared
in vivo
imaging. Moreover, 12 h post-injection, PDT
experiments (635 nm laser, 500 mW/cm
2
) have been realized with success, inducing
a necrosis process.
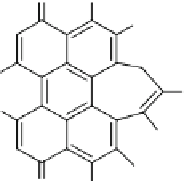
Yang and collaborators (
2010
) prepared MSN encapsulating hypocrellin B (HB,
Fig.
14
) as a PDT agent and coated with a lipid layer. The particles were prepared
using a base-catalyzed sol-gel method. The particles were calcinated and the HB
were impregnated in the particles. A coat of lipids (1-palmitoyl-2-[6-[7-nitro-2-1,3-
benzoxadiazol-4-yl)amino]hexanoyl]-
sn-
glycero-3-phosphocholine, 1,2-dimyris-
toyl-
sn
-glycero-3-phophocholine and 1,2-dimyristoyl-
sn
-glycero-3-phosphate
monosodium salt) was prepared by drying the mixed lipid solution and reconstituting
it as vesicles following a conventional method. The vesicles were then adsorbed
O H
OMe
MeO
MeO
Me
COCH
3
OMe
O
OH
Fig. 14
Hypocrellin B (HB)
Search WWH ::

Custom Search