Biomedical Engineering Reference
In-Depth Information
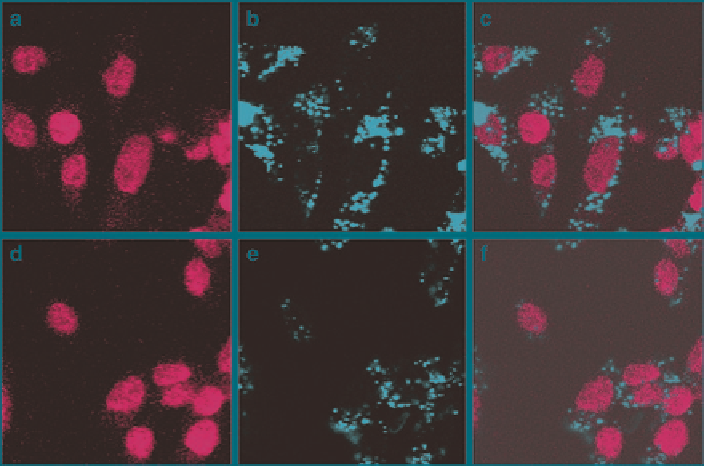
Fig. 8
Fluorescence confocal micrographs of CHO cells incubated with 50 g mL − 1 Tubular-
FITC-MSN (
a
-
c
) and Spherical-FITC-MSN (
d
-
f
). (
a
and
d
) Fluorescence image excited at
340 nm to visualize the cell nuclei stained with DAPI. (
b
and
e
) Fluorescence image excited at
488 nm to visualize the FITC doped MSN that have been internalized by cells. (
c
and
f
) Overlaid
micrographs of (
a
and
b
) and (
d
and
e
), respectively. (Reprinted from Trewyn et al.
2008
,
Copyright (2008), with permission from Elsevier)
internalized at all, but this size limit is cell line dependent. Overall, it is better to
have small round particles, sufficiently stabilized to avoid the formation of large
aggregates.
4.2.2
Influence of Surface Charge
Recent studies (Slowing et al.
2006
) have shown that the uptake of positively
charged mesoporous silica nanoparticles was more efficient than the uptake of
negative particles, due to the net negative charge of cell membranes. These studies
have also enlighten that negatively charged particles were more able to escape
endosomes within 6 h, probably due to the “proton sponge effect” that supposes
that the more negatively charge particles have a better buffering capacity which
is a key factor for endosomal escape. Thus both factors must be taken into
account while designing silica-based drug delivery systems. Another point is that
positive nanoparticles are more subject to non-specific adhesion of proteins or
non-specific interaction with the cells. But surface charge is not the only tunable
parameter and different groups can be grafted on the surface of the silica-based
Search WWH ::

Custom Search