Biomedical Engineering Reference
In-Depth Information
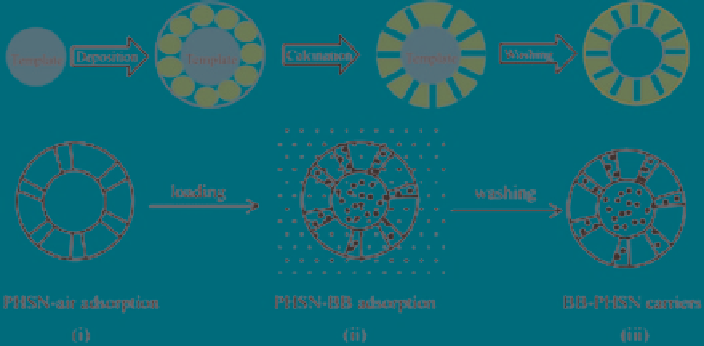
Fig. 7
Schematic illustration of the preparation of porous hollow silica spheres (HSN) and further
immobilisation of
Brilliant Blue
(BB). (Reprinted from (Li et al.
2004
), Copyright (2004), with
permission from Elsevier)
4
Intracellular Uptake and Fate of Silica-Based Nanoparticles
One of the interests of silica-based drug delivery system is the possibility to control
and manipulate intracellular uptake of the particles and their accumulation for an
extended period of time. To achieve such a goal the versatile chemistry of silica is
exploited to graft several functional groups such as stimuli-responsive gatekeepers
to control the release of the loaded drug, cell-specific moieties to achieve a targeted
delivery, cell-penetrating peptides to enhance the uptake efficiency, protecting polymer
layers, or ligands for endosomal escape strategies…(Vivero-Escoto et al.
2010b
).
All of these modifications are aimed at obtaining an efficient drug delivery system,
which means that the device must be able to: (i) reach its target without being pre-
maturely eliminated (role of surface coating), (ii) be internalized (functionalization
for enhanced endocytosis or direct uptake), (iii) reach the right location (cellular
and intracellular targeting) and (iv) present no toxicity for the healthy cells (control
of the long-term fate of nanoparticles).
4.1
Avoiding Premature Elimination
As mentioned earlier, coating silica nanoparticles has proved to be useful to
enhance systemic circulating half-time. Some polymers such as PEG (polyeth-
ylene glycol) or carbohydrates can be used for this purpose because it creates a
protective layer (Mailaender and Landfester.
2009
). The polymer shell created
on the surface of the particles makes them more hydrophilic and decreases
unspecific adsorption of proteins. If the particles have no opsonising proteins
adsorbed on the surface they are not recognized and eliminated by macrophages.
Search WWH ::

Custom Search