Biomedical Engineering Reference
In-Depth Information
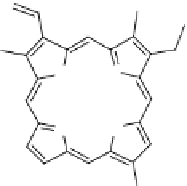
Fig. 19
Chemical structure
of the photosensitizer
Chlorine e6
NH
N
N
HN
HO
OH
O
HO
O
O
which has been used in photodynamic therapy (PDT) because upon light exposure
Ce6 generates highly reactive oxygen species (ROS) including free radicals and/or
singlet oxygen
1
O
2
. In the core of a lipid bilayer, carbon-carbon double bonds of
unsaturated lipids have been shown to be privileged sites for
1
O
2
and radical reac-
tions that lead to cellular membrane alteration. The selected photo-inert copolymer
is again the prototypical PEO-
b
-PBD, which contains a large number of carbon-
carbon double bonds that are preferred sites of photo-oxidation. Ce6 is an
amphiphilic molecule and can be loaded in the membrane as well as in the aqueous
internal compartment. In these polymersomes loaded with Ce6, we have observed a
complex sequence of light-induced morphological changes. Using micromechanical
assays based on micropipette manipulation, we have quantitatively monitored the
different phases of the photo-response, which include membrane area variation,
osmotic swelling, membrane cross-linking and vesicle deflation. In addition, these
morphological changes can be adjusted by the concentration of Ce6 (see Fig.
20
).
We have thus gained insight into the complex cascade of chemical reactions involved
in photosensitization. Our findings suggest that composite chlorine-copolymer vesi-
cles may be used as a new class of light-sensitive drug carriers.
3.3
Polymersomes Responsive to Magnetic Field
The magnetic field is a very promising stimulus in drug delivery, because it is easy
to be applied remotely and permits also to combine together the medical diagnosis
and therapy due to the development of MRI (magnetic resonance imaging) tech-
nique. The common strategy is to incorporate magnetic nanoparticles (e.g., g-Fe
2
O
3
)
into the vesicle structures. Iron oxide nanoparticles, namely magnemite (g-Fe
2
O
3
)
or magnetite (Fe
3
O
4
), with particle sizes of 4-10 nm have drawn special interest for
biomedical applications. They are referred to as superparamagnetic iron oxide
nanoparticles (SPIONs) due to their superparamagnetic properties and small size.
The resulting superparamagnetic vesicles have potential applications because of the
specific properties of magnetic iron oxide in the fields of biomedicine and biotech-
nology: e.g., manipulation by an external magnetic field gradient, radio-frequency
heating for cancer therapy, and labelling of organs in magnetic resonant imaging.






Search WWH ::

Custom Search