Biomedical Engineering Reference
In-Depth Information
3.1
Homopolymerization and Random Copolymerization
Homopolymers from alkyl cyanoacrylates (Fig.
3
) has been well-studied since the
late 1970s (Donnelly et al.
1977
; Johnston and Pepper
1981a, b, c
). The polymer-
ization of ethyl cyanoacrylate (ECA) and
n
-butyl cyanoacrylate (
n
BA) was under-
taken in tetrahydrofuran (THF) and initiated either by simple anions or by organic
bases, leading to anionic or zwitterionic mechanism, respectively (Donnelly et al.
1977
). Multiple parameters were varied in order to investigate their influence on the
macromolecular properties of the resulting polymers (Pepper
1980
; Johnston and
Pepper
1981a, b, c
; Pepper and Ryan
1983
; Cronin and Pepper
1988
; Eromosele
et al.
1989
; Johnson et al.
1995
).
In particular for zwitterionic polymerization of
n
BCA, the influence of the
nature of the initiator, the inhibiting species and the presence of water on the mac-
romolecular characteristics of the polymer and on the polymerization kinetics was
thoroughly investigated using several covalent organic bases (Pepper
1980
;
Johnston and Pepper
1981a, b, c
; Pepper and Ryan
1983
; Cronin and Pepper
1988
;
Eromosele et al.
1989
). Regarding the anionic mechanism, several tetrabutyl
ammonium salts were used as initiators for the polymerization of
n
BCA in THF
and the best results were obtained from the hydroxide-based one (Eromosele and
Pepper
1986, 1989a, b
).
Copolymerization between cyanoacrylate derivatives, namely ECA and
n
BCA,
was also performed in order to tune the glass transition temperature of the resulting
materials. Copolymerizations were performed either by a piperidine-catalyzed bulk
polymerization, leading to transparent brittle films, or by polymerization in aqueous
medium in the presence of sodium bicarbonate, in order to obtain white powders
(Denchev et al.
2008
).
When a suitable inhibitor is introduced in the reaction medium (such as boron
trifluoride-acetic acid complex, propane-1,3-sultone or acetic acid), anionic
polymerization is made negligible during the timescale of the polymerization and
allow a free-radical mechanism to be the main chain-extension process (Canale
et al.
1960
; Kinsinger et al.
1965
; Otsu and Yamada
1967
; Bevington et al.
1976
;
CN
CN
CN
CN
CN
CN
O
O
O
O
O
O
O
O
O
O
O
O
14
6
OCA
MCA
ECA
n
BCA
IBCA
HDCA
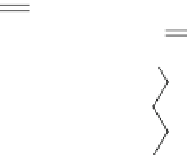
Fig. 3
Structure of methyl cyanoacrylate (MCA), ethyl cyanoacrylate (ECA),
n
-butyl cyanoacrylate
(
n
BCA), isobutyl cyanoacrylate (IBCA), octyl cyanoacrylate (OCA) and hexadecyl cyanoacrylate
(HDCA)




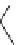



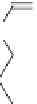




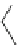





Search WWH ::

Custom Search