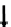Biology Reference
In-Depth Information
increased blood flow due to dilation of arterioles, which causes chemotaxis of neu-
trophils and adherence to the vascular wall
[7]
(
Figure 8.1
).
Perhaps the most potent mediator of neurogenic inflammation is SP. Moreover,
SP is the most abundant neuropeptide in the CNS, being widely distributed in the
MIND
Access to brain via
circumventricular region
area postrema
Systemic cytokine
production
Spinal reflex
Efferent
vagus
HPA-axis
SNS
Vagus afferents
Somatic nerves
Paraganglia
Spillover to blood
Gluco-
corticoids
Catechol-
amines
Acetyl-
choline
Substance P,
Calcitonin gene-
related peptide
Direct stimulation
IL-1
↑
IL-6
↑
TNF-
α↑
Immunological
inflammation
Indirect stimulation
Immune cells
Tissue cells
Neurons
Immune cells
Tissue cells
IL-10
↑
TGF-
β ↑
IL-1
↓
TNF-
α↓
IL-12
↓
Inflammatory signal
Bacterial material, tissue injury, chemical toxin
Anti-inflammatory response
Neurogenic inflammation
Figure 8.1
This diagram demonstrates the pathways through which the immune and nervous
systems act together to eliminate an inflammatory stimulus. In this situation the type of
response—neurogenic or immunological—depends on the type of the signal. Chemical toxins
predominantly induce a neurogenic inflammation via a spinal reflex. The subsequent release
of neuropeptides induces vasodilation and capillary leakage leading to the characteristic
symptoms of redness, swelling, and pain. In contrast, bacterial material and tissue debris
induce an immunological inflammation with the local release of cytokines. However,
these cytokines may affect somatosensory nerve endings and transmit the information
about inflammation into the brain, where it will be processed and a brain-mediated anti-
inflammatory response may be initiated. In this scenario, the HPA axis, the SNS, and the
efferent vagus will be activated. This leads to the release of glucocorticoids, catecholamines,
and acetylcholine. These very potent anti-inflammatory mediators induce the production of
anti-inflammatory cytokines in both immune and nonimmune cells. The goal of this reaction
is to confine inflammation locally and to prevent spillover of cytokines into the general
circulation. However, if this mechanism does not succeed, a systemic inflammatory syndrome
may occur with cytokines in the blood. These blood cytokines may directly access the brain
via the circumventricular region or the area postrema. This informs the brain about a situation
that is more severe than a local inflammation, and may lead to an alarm situation that results in
amplification of the brain-mediated anti-inflammatory response syndrome.