Environmental Engineering Reference
In-Depth Information
two hydrothermal systems in Nevada (Smith et al., 2005).
The authors measured deviations ranging from
Meteorites
Nevada
Idrija
Various
3.5 to
Cinnabar
1.3‰ for meta-
cinnabar, while the underlying rock showed values of
0.4‰ for cinnabar and from
1.4 to
Nevada
Idrija
2.1‰. They explained the observed light iso-
tope enrichment in cinnabar relative to meta-cinnabar
and the underlying veins through a combined process of
boiling hydrothermal fl uid, surface oxidation, and kinetic
effects during mineral precipitation. A similar fraction-
ation between cinnabar and meta-cinnabar was observed
in ores from an Hg mine in Idria, Slovenia (Foucher et al.,
2009), where
0.2 to
Meta-cinnabar
Nevada
Bolivia
Rocks
Coal (USA)*
Au-mine
San Francisco Bay
Minamata
Idria/Gulf of Trieste
Bolivia*
Sediments
0.23‰ for black cin-
nabar was measured. This relatively larger range of isotope
deviation in related ores points to the importance of tem-
perature causing boiling, evaporation, and precipitation of
Hg phases in some deposits, while other deposits such as
Almadén, Spain are low-temperature systems, in which
boiling is not common. Those deposits are expected to
show more uniform
0.26‰ for red and
L. Michigan**
New England**
Bolivia**
Fish
Hair**
Vulcanic Hg(0)
Arctic snow
δ
202
Hg.
-4
-3
-2
-1
0
1
2
3
4
δ
202
Hg (‰)
Meteorites
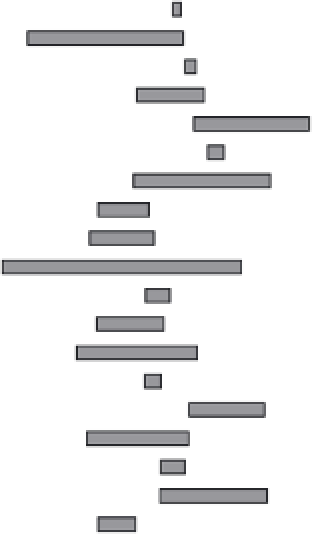
FIGURE 4.1
Reported ranges of Hg isotope composition for
environmental samples, analyzed by MC-ICP/MS and expressed
relative to NIST 3133 (vertical line at 0 ‰). Mass-independent
fractionation has been reported for some terrestrial (*) and many
biological (**) samples.
Most Hg isotope ratio measurements of meteorites, mostly
chondrites, were conducted with techniques other than
MC-ICP/MS. Because of the associated larger uncertainty
with these methods, many of the older data are now consid-
ered suspect. MC-ICP/MS measurements were conducted to
determine the isotope composition in the Murchison and
Allende carbonaceous chondrites (Lauretta et al., 2001). In
contrast to earlier NAA studies, this newer study did not
fi nd anomalous deviations from terrestrial values. Instead,
the bulk isotope composition did not deviate statistically
from their reference standard (UM-Almadén), suggesting
Hg as well. Depending on the location of the sample, mea-
sured Hg isotope ratios varied greatly between the source
Hg at the mine (
0.15 to
0.05‰) and the background Hg
in river sediments (
2.75‰) (Foucher and Hintel-
mann, 2004). Based on these data, a simple mixing model
was developed to estimate the proportion of discharged Hg
in the total Hg measured downstream of the mine (Foucher
and Hintelmann, unpublished).
2.83 to
δ
202
Hg in Minamata Bay
0.54‰ for Allende and Murchinson Hg. In contrast to
earlier NAA studies, no anomalous
spans from
1.0‰, depending on the location in
the bay. Samples collected in the Northern San Francisco
Bay area showed little variation (
2.0 to
δ
202/196
Hg fractionation
was found.
1.0‰). Another
study determined Hg isotope variations in Arctic lake sedi-
ments (Jackson et al., 2004). The authors reported data for a
dated sediment core from the anoxic zone of Romulus Lake,
a small oligotrophic, saline and meromictic lake in the
high arctic desert of Ellesmere Island, Nunavut, Canada,
and found that deeper (older) sediments were enrichment
with lighter isotopes. They hypothesize that the observed
Hg isotope fractionation was caused by microbial activities
linked to redox processes in the lake. However, they also
do not rule out that the measured Hg isotope ratios may be
indicative of different Hg sources in the lake.
1.1 to
Coal
Since coal burning is one of the main ways that Hg is intro-
duced into the atmosphere, the Hg isotope ratio signature
of coal is of great interest for studies trying to track Hg
from sources to receptor sites. However, one early investiga-
tion was unable to differentiate Hg isotope ratios in coal—
Evans et al. (2001) found variations between
1.5‰ and
0‰ relative to NIST 1632b. Unfortunately, the precision of
the isotope ratio determination for the reference material
was less than 2‰ (RSD) at the time, which did not allow
conclusive differentiation between coal samples. Neverthe-
less, considering that analytical techniques have matured
greatly since then, it is worthwhile to revisit the question
with optimized methods. More recent work indicated
signifi cant Hg isotope abundance differences in coal from
various locations in the United States, China, and Kazakh-
stan, with
Ores
Few data have been reported for Hg isotope ratios in Hg
ores. One study (Hintelmann and Lu, 2003) compared
cinnabar ores from many different locations and detected
deviations between
1.3‰ and 0.00‰. Another study
measured Hg isotope ratios in materials obtained from
δ
202
Hg from
0.9 to
3.0‰ and
201
Hg from
0.1 to 0.4‰ (Biswas et al., 2008).