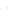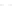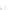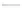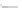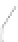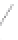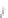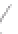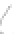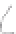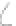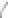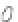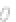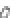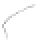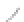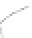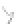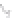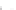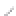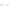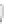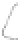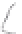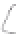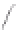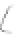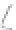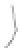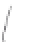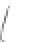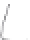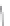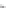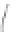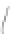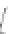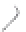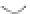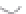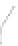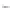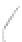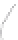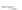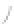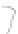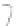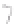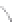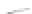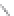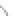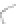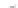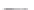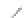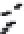Environmental Engineering Reference
In-Depth Information
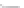
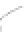
Fluoropolymer vials
Hg free
N
2
or Ar
Distillate
Sample +
reagents
Aluminum
heating block
125°C
Ice bath
FIGURE 3.3
Schematic diagram of the distillation system used to separate monomethylmercury from complex matrices prior to the
derivatization step. (
Source:
EPA. 1998b.)
about 0.3 ng/L at typical sample volumes (Rapsomanikis
and Craig, 1991).
This method has been published by the EPA as Method
1630 (EPA, 1998b) and has frequently been adopted in
laboratories involved in studies of the biogeochemical
cycle of Hg. EPA Method 1630 remains in draft status and
has not yet been fully promulgated by EPA.
ORGANO-MERCURY DETERMINATIONS USING
AN EXTRACTION STEP
In a typical extraction method, MMHg halide (Br
, Cl
or I
) is extracted into an organic solvent (benzene or
toluene) after acidifi cation. This is followed by derivatiza-
tion to a water-soluble adduct of methylmercury-cysteine,
which is extracted into the aqueous phase. After acidifi -
cation, CH
3
HgX (X is a halide ion) is back-extracted into
a small amount of organic solvent. An aliquot is then
injected onto a gas-liquid chromatography (GLC) column
and detected by electron capture detection (ECD) or any
other suitably sensitive detector (such as a plasma emis-
sion detector). Packed or capillary columns can be used
as described later for MMHg determination in other envi-
ronmental samples.
Craig (1986) has reviewed the many modifi cations to this
extraction procedure. For example, the MMHg compound
may be transferred into organic solvent as dithizonates fol-
lowed by clean-up steps and detection by GC-ECD (Akagi
and Nishimura, 1991). Inorganic and organic Hg species
can be preconcentrated on dithiocarbamate or sulfhydryl
cotton-fi ber adsorbent that is then extracted as described
above (Lee and Mowrer, 1989; Jones et al., 1995). However,
in some water samples, artifact formation of MMHg was
observed during solid-phase extraction of water samples
(Celo et al., 2004). The common drawbacks of most of
these extraction procedures are the large sample require-
ments, low extraction yields, and nonspecifi c separation of
dimethylmercury, if present.
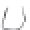
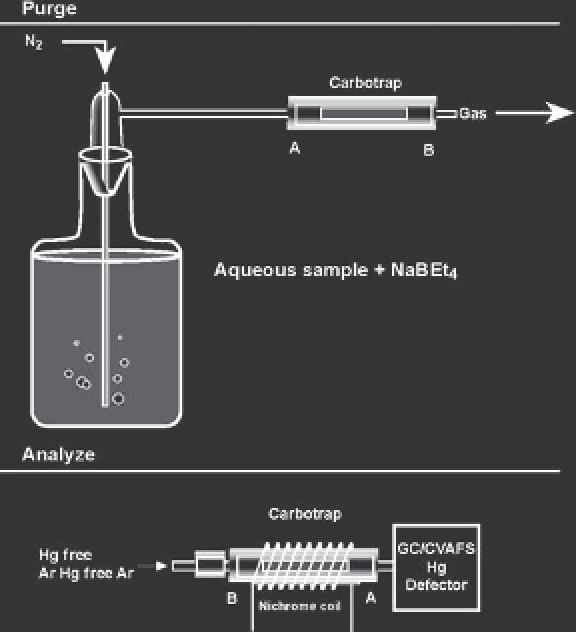
FIGURE 3.4
Schematic diagram of the purging system to strip
derivatized mercury species from solution onto an absorbent
material. NaBEt
4
sodium tetraethylborate. (
Source:
EPA. 1998b.)
materials such as Carbotrap or Tenax at room temperature
(Figure 3.4).
Subsequent separation of the various Hg species is
then conducted by heating the adsorbed Hg species off
of the collection column and into a GC column. After
thermal release, individual Hg compounds are separated
by cryogenic or isothermal GC. As the species are eluted
they are thermally decomposed (pyrolyzed) at high
temperature (
750°C) and are quantifi ed as Hg
0
using a
CVAFS detector that achieves very low detection limits
(
10 pg, equating to about 0.02 ng/L at typical sample
volumes) (Figure 3.5). A CVAAS detector can also be
used, but its detection limit is much higher, equating to