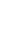Environmental Engineering Reference
In-Depth Information
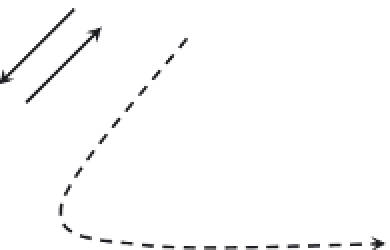
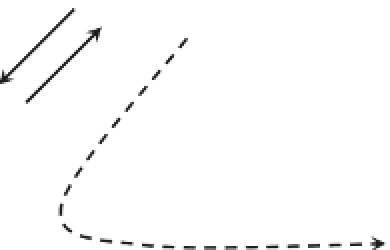
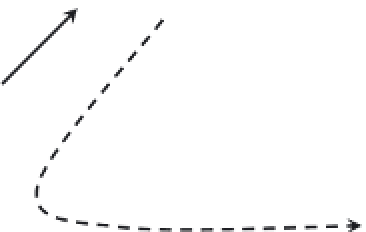
FIGURE 3.2
Competition for the partitioning of the free mercury ion into various pools or fractions in natural waters.
This removes any matrix interferences with the analysis
that result in a biased determination and allows the detec-
tion method to quantify the mercury.
Finally, because Hg is ubiquitous in the environment and
is used in so many chemical manufacturing processes, fi nd-
ing suitably clean reagents for sample preparation or diges-
tion steps can be diffi cult. For low-level aqueous mercury
measurements, it is essential, therefore, that all reagents
used be rigorously checked for Hg contamination prior to
use and that all laboratory ware that comes in contact with
the sample be of appropriate materials and be rigorously
cleaned to maintain contamination at suitably low levels
(Gill and Fitzgerald, 1985, 1987; Bloom, 1994; Parker and
Bloom, 2005).
aqueous samples over the past 15 years (Gill and Fitzger-
ald, 1985, 1987; Bloom, 1995; Fitzgerald, 1999; Parker and
Bloom, 2005).
As noted previously, the total Hg in a water sample can
be composed of several distinct forms or pools, includ-
ing “dissolved” Hg (usually operationally defi ned as that
mercury passing through a 0.45-µm fi lter), Hg associated
with particulate and colloidal matter, volatile elemental
Hg
0
, and labile (or reactive) Hg(II). All these forms can all
be quantifi ed as long as the samples are collected and pre-
served properly for the species to be determined.
SAMPLING AND STORAGE
Collection and handling of aqueous samples for low-level
determination of Hg must address several factors, includ-
ing whether or not the sample is representative, possible
interconversion processes, contamination, and preserva-
tion and storage of the matrix before analysis. The mea-
surement (sampling and analysis) protocol must be care-
fully designed if speciation of Hg forms in the aqueous
samples is intended. The stability of Hg in solution is
affected by many factors, including: (a) the concentration
of Hg and its compounds, (b) the type of water sample, (c)
the type of containers used, (d) the cleaning and pretreat-
ment of the containers, and (e) the preservative added.
Table 3.3 lists recommended sample-collection containers,
hold times, and preservation methods for the most com-
mon environmental samples collected for inorganic or
total Hg analysis.
The best materials for sample storage and sample pro-
cessing are Pyrex and silica (quartz) glass or Tefl on
Total Mercury and Inorganic Mercury
Species in Water
Recent improvements in analytical methods have dem-
onstrated that much of the historical data for total Hg
in environmental water samples collected prior to the
early 1990s was biased, either high because of contami-
nation during sampling and analysis or low because of
improper sample collection containers or improper pres-
ervation techniques (Fitzgerald, 1999). Problems arising
in the analysis of total Hg in natural water samples are
not connected with the fi nal measurement, but rather
with diffi culties associated with contamination-free sam-
pling and losses due to volatilization and adsorption dur-
ing storage. There have been remarkable improvements
in sampling and analytical techniques that have resulted
in a dramatic increase in the reliability of data for Hg in