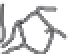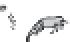Environmental Engineering Reference
In-Depth Information
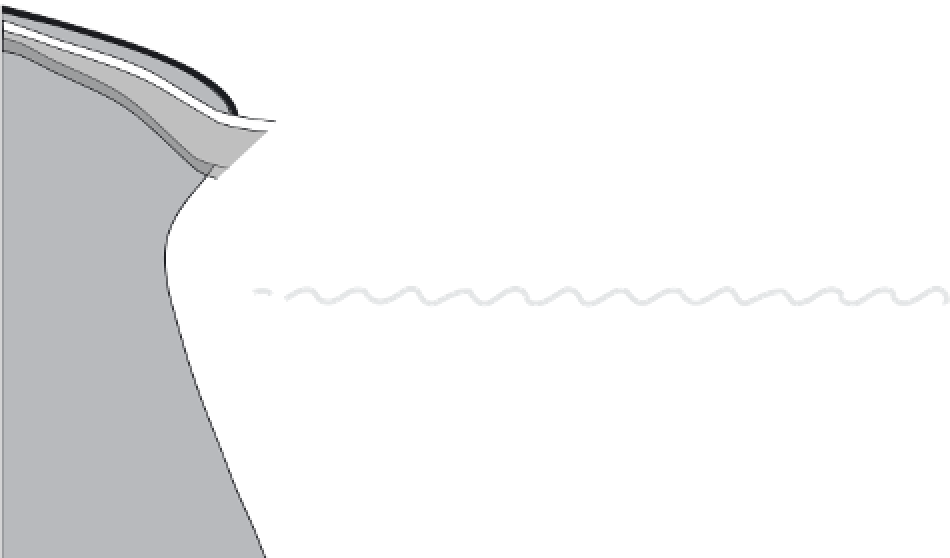
ATMOSPHERE
Fishing (-0.04)
Volatilization (-?)
h
ν
Atmospheric
Deposition (+0.02)
Particle scavenging
Photo-degradation (-0.7)
LAND
Estuary sediments
Rivers, waste water,
estuaries (+0.21)
Biotic methylation
in water column (+?)
Abiotic methylation
in water column (+?)
Coastal
wetlands(+?)
Methylation in particle
microenvironments (+?)
Biotic demethylation
in water column (-?)
Abiotic demethylation
in water column (-?)
Submarine
groundwater
discharge
(+0.004)
DMHg
MMHg (+?)
OCEAN WATER COLUMN
Oceanic Reservoir
70 Mmol MMHg
Coastal and
Coastal and
shelf sediment
Coastal and
shelf sediment
benthic flux (+0.18)
shelf sediment
benthic flux (+0.18)
benthic flux (+0.18)
Geothermal
inputs (+0.2)
MARINE
SEDIMENTS
Deep sea sediment
Deep sea sediment
benthic flux (+0.6)
Deep sea sediment
benthic flux (+0.6)
Sediment burial (-0.21)
Sediment burial (-0.21)
benthic flux (+0.6)
FIGURE 10.7
Preliminary mass balance of MMHg in the ocean. Estimates of all sources and sinks are derived and presented in the text. Many
of the fl uxes are estimated from very limited data of direct measurements, and thus uncertainties for some fl uxes are likely to be as great as an
order of magnitude. This is particularly true of the deep sea MMHg fl uxes, the magnitude of which is inconsistent with fi eld measurements,
which do not show particularly elevated MMHg concentrations in the deep ocean.
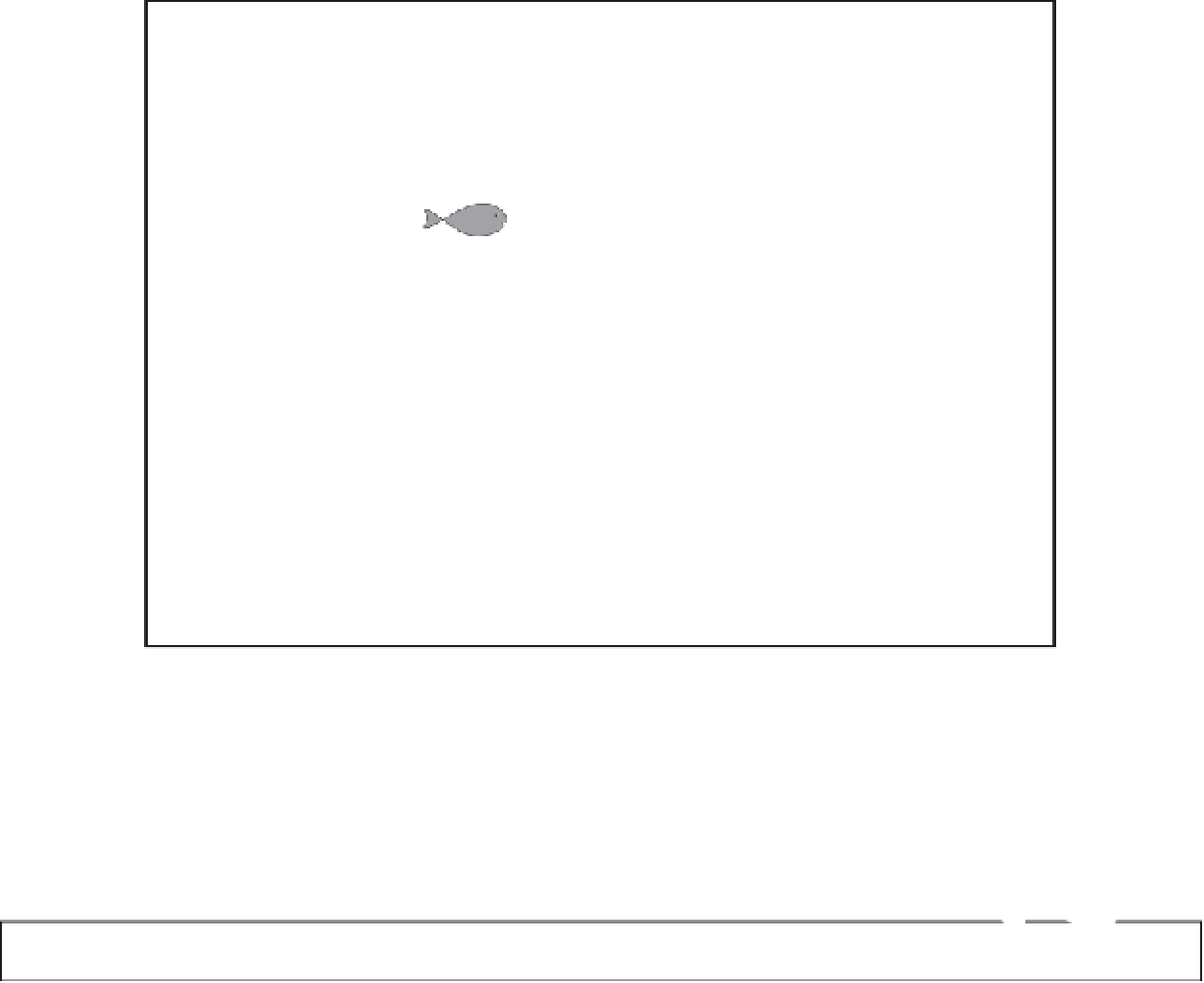
100,000x
2-5x
2-5x
2-5x
Water
Algae
Zooplankton
Prey Fish
Predator Fish
FIGURE 10.8
Typical magnitude of mercury bioaccumulation and biomagnifi cation in a stylized aquatic food chain.
and Wong, 2003; Watras and Bloom, 1992). Those reports
demonstrate that in most cases MMHg is the only form of
mercury biomagnifi ed and that MMHg constitutes a larger
fraction of the overall body burden of mercury in aquatic
organisms with increasing trophic level.
effectively transferred to, and bioaccumulated by, zoo-
plankton and planktivorous fi sh than inorganic mercury
(Mason et al., 1996; Pickhardt and Fisher, 2007).
Because of their effi cacy in sequestering MMHg from
surface waters, it has been proposed that mercury concen-
trations in phytoplankton, on a per-cell basis, decrease dur-
ing algal blooms ( Chen and Folt, 2005; Pickhardt et al.,
2002), potentially leading to reduced bioaccumulation
of mercury to higher trophic levels during blooms. Such
studies were initially conducted in freshwaters, but a study
in San Francisco Bay found similar results (Luengen and
Flegal, 2009).
Mercury concentrations in those and other
in situ
phy-
toplankton populations are not usually measured directly
because of the concurrent presence of bacteria, detritus,
and suspended sediments also collected in phytoplankton
tows. Instead, concentrations of mercury in phytoplankton
are often derived by normalizing mercury levels to other
parameters (e.g., aluminum as a measure of suspended sedi-
ments; chlorophyll as a measure of primary productivity and
standing biomass). While such methods enable calculations
Mercury in Phytoplankton
Numerous studies have demonstrated the important role
that phytoplankton play in the introduction of mercury
into aquatic food chains. These include laboratory (Mason
et al., 1996; Moye et al., 2002), mesocosm (Pickhardt et al.,
2002), and fi eld studies (Chen and Folt, 2005; Kainz and
Mazumder, 2005; Watras and Bloom, 1992). Those stud-
ies have shown that phytoplankton generally concen-
trate MMHg 10
4
-10
5
above ambient water concentrations
(Moye et al., 2002; Pickhardt and Fisher, 2007; Watras
et al. 1998), and this accumulation represents the largest
relative increase in MMHg levels at any point in a food
web. Because most of the MMHg in phytoplankton is in the
cytoplasm, rather than sorbed
onto the surface, it is more