Environmental Engineering Reference
In-Depth Information
higher total mercury concentrations are found in contami-
nated coastal and estuary environments, where they can
exceed 500 pM (Balcom et al., 2008; Conaway et al., 2003;
Faganeli et al., 2003; Heyes et al., 2004). Such trends are
not surprising, given that estuaries and coastal zones are
highly infl uenced by industrial and other human activi-
ties, including mercury pollution.
The higher mercury concentrations in the nearshore are
also the result of the greater importance of terrestrial run-
off as a source of mercury to coastal waters relative to the
open ocean, and are thus controlled by sediment transport
and resuspension. Inorganic mercury in the water column
tends to be associated with particulate matter, with particle
distribution coeffi cients (K
d
) typically in the range 10
5
-10
6
for estuary and coastal waters (Baeyens et al., 1998; Balcom
et al., 2008; Benoit et al., 1998; Choe et al., 2003; Conaway
et al., 2003; Coquery et al., 1997; Laurier et al., 2003a;
Leermakers et al,. 1995, 2001; Stordal et al., 1996). As a con-
sequence, most transport of mercury within aquatic systems
occurs via the particulate phase, and approximately 90% of
the riverine inputs of mercury to the ocean are deposited in
coastal sediments (Sunderland and Mason, 2007). Estuaries
are sinks for inorganic, particle-associated mercury, but can
be net sources of MMHg (Benoit et al., 1998; Faganeli et al.,
2003; Macleod et al., 2005; Mason et al., 1999).
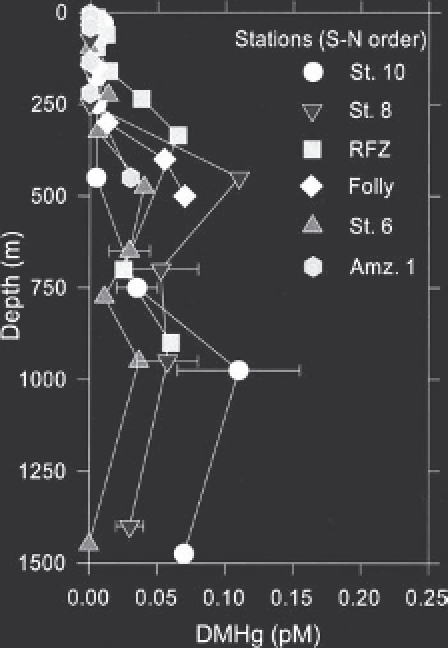
FIGURE 10.3
Depth profi les of DMHg to 1500 m in the South and
equatorial Atlantic measured in 1996 (Mason and Sullivan, 1999). See
fi gure 10.2 for map with station locations. RFZ
Romanche Fracture
Zone; Amz.
Amazon. (Reprinted, with permission, from Elsevier.)
(Dyrssen and Wedborg, 1991; Mason et al., 1996; Amirbahman
et al., 2002). DMHg is present as a dissolved gas in saline
waters, and it is the predominant methylated form of mercury
in much of the open ocean below the thermocline (Cossa
et al., 1994, 1997; Horvat et al., 2003; Mason and Fitzgerald,
1993; Mason et al., 1995, 1998; Mason and Sullivan, 1999;
St. Louis et al., 2007). Exceptions to this trend are in surface
waters, many coastal environments, and the Mediterranean
Sea, all areas where MMHg concentrations are generally
greater than DMHg concentrations. DMHg is found at depth
in the water column at femtomolar to subpicomolar concen-
trations, but is rarely detectable in the mixed layer (Pongratz
and Heumann, 1998). Depth profi les for DMHg in the equato-
rial and South Atlantic are shown in Figures 10.2 and 10.3.
DMHg has generally not been detected in the coastal zone
waters, with the exception of high Arctic marine surface
waters (St. Louis et al., 2007; Kirk et al., 2008) and upwelled
coastal waters off California (Conaway et al., 2009). Water
column concentrations of DMHg and MMHg, and the per-
centage of mercury existing in a methylated form, are sum-
marized in Table 10.1 for various oceanic locations.
Mercury in the Open Ocean
The distribution and mass balance of total mercury in the
water column of the oceans have been reviewed elsewhere
(Lamborg et al., 2002; Mason and Sheu, 2002; Laurier et al.,
2004; Fitzgerald et al., 2007; Strode et al., 2007; Sunderland
and Mason, 2007; Selin et al., 2008). Typical depth profi les
of total mercury in the North Pacifi c and tropical South
Atlantic are shown in Figures 10.1 and 10.2, respectively.
They illustrate vertical variations and the distribution of
total mercury in the open ocean, which are controlled by
the magnitude of different sources and sinks at the bound-
aries of the marine environment, as well as water column
processes. These include particle scavenging, remineraliza-
tion of sinking particles, sediment diagenesis, water-col-
umn stratifi cation, vertical mixing, ventilation, and hori-
zontal advection along isopycnals (Laurier et al., 2004).
The principle sources and transport processes of total
mercury to the open ocean are: (1) atmospheric deposition
and (2) runoff from coastal and freshwater systems fol-
lowed by advection offshore (Lamborg et al., 2002; Mason
and Sheu, 2002; Sunderland and Mason, 2007). Mercury
from hydrothermal vents, submarine volcanic activity,
and weathering of oceanic crust and other submarine geo-
logic structures are additional potentially important natu-
ral sources, but their contributions are not well known. A
global mass balance developed by Sunderland and Mason
(2007) estimating the magnitude of important fl uxes of
total mercury between environmental reservoirs for both
preindustrial times and the present is shown in Figure 10.4
Mercury in Estuarine and Coastal Waters
Mercury concentrations are higher in coastal and estuary
waters than in the open ocean, with unfi ltered total mer-
cury concentrations in the nearshore typically within the
range 1-40 pM (Baeyens et al., 1998; Benoit et al., 1998;
Coquery et al., 1997; Cossa et al., 1997; Laurier et al., 2003a;
Leermakers et al., 1995, 2001; Mason et al., 1999; Rolfhus
and Fitzgerald, 2001; Stordal et al., 1996). Considerably