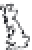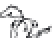Environmental Engineering Reference
In-Depth Information
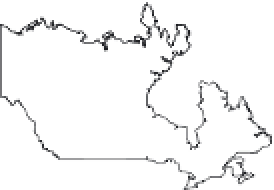
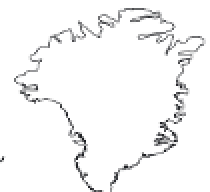
FIGURE 8.1
Global sites of terrestrial mercury cycling research cited in this chapter.
In general, more than 80% of annual atmospheric Hg
deposition to terrestrial watersheds is retained in the soil
(Aastrup et al., 1991; Lee et al., 1998; Hintelmann et al.,
2002). Soil retention has protected the aquatic ecosystem
from receiving the full load of anthropogenic Hg deposi-
tion. Conversely, however, soil Hg retention has increased
the store of Hg in soils and vegetation, posing an uncer-
tain but potentially large future risk (Harris et al., 2007).
Annual losses of Hg through volatilization or streamfl ow
represent only a minute fraction of the catchment store.
Decreased Hg emissions and deposition appear to result in a
direct reduction of Hg uptake in aquatic ecosystems (Evers
et al., 2007; Harris et al., 2007), but decreased emissions may
not be effective at reducing the terrestrial outputs of Hg
and MeHg. Thus, management for Hg in freshwater ecosys-
tems becomes a much more complex problem, in that not
only direct atmospheric deposition of Hg, but the entire
Hg-contaminated landscape, is a Hg source. Indeed, one
of the paramount issues facing scientists and policymakers
today is the ultimate fate of the large amount of “legacy
Hg” that has accumulated in terrestrial soils and vegeta-
tion. Prudent land management can help to limit Hg in
runoff.
Terrestrial Hg cycling is important for several reasons:
(1) terrestrial vegetation enhances atmospheric Hg capture;
Hg deposition to forests may be 3-4 times greater than Hg
deposition to adjacent water bodies (Miller et al., 2005);
(2) despite the high Hg retention in terrestrial landscapes,
Hg “leakage” from land areas often results in terrestrial Hg
being the dominant source to a water body; (3) a signifi cant
proportion of the MeHg in freshwaters forms in the terres-
trial landscape, notably in wetlands but also in upland soils,
before its hydrologic transport to water bodies; (4) Hg export
from watersheds tends to be episodic, and these high-Hg
pulses may either stimulate methylation or comprise a
major source of MeHg in their own right; and (5) there is
increasing documentation of Hg bio-accumulation in ter-
restrial food webs (Evers et al., 2007) as well as the potential
of direct effects on soil microbial communities.
Although inorganic total Hg (THg) is the main form of
Hg in atmospheric deposition, the dominant form in fi sh
is MeHg. The transformation of THg to MeHg occurs nat-
urally in anoxic environments—that is, water-saturated
zones in peatlands, riparian areas, and sediments (Meili,
1997; Holmes and Lean, 2006). Under humid hydrologic
regimes, such zones are naturally abundant (e.g., wetlands
in boreal and tropical regions), or they can be created/
induced by land-use changes such as forest harvest or
fl ooding from hydroelectric dams and reservoirs. Advances
have been made in localizing and identifying processes in
the forest fl oor and near-stream wetland areas that increase
the loading of bio-available MeHg to the aquatic ecosystem
(Lee et al., 2000; Branfi reun and Roulet, 2002; Mitchell
et al., 2008b). Water-table fl uctuations may also stimulate
the sulfur-reducing bacteria (SRB) which are particularly
effective in methylating Hg (Sorensen et al., 2005; Selch
et al., 2007). Sulfur (S) deposition may enhance methylation
by SRB as well (Branfi reun et al., 2001; Jeremiason et al.,
2006; Drevnick et al., 2007; Mitchell et al., 2008a). These
fi ndings help to explain the observations that catchment
disturbance, such as forestry operations and land develop-
ment, increase the MeHg exported from catchments (Porvari
et al., 2003) as well as the amount of Hg bio-accumulated
in downstream fi sh (Garcia and Carignan, 2005).