Environmental Engineering Reference
In-Depth Information
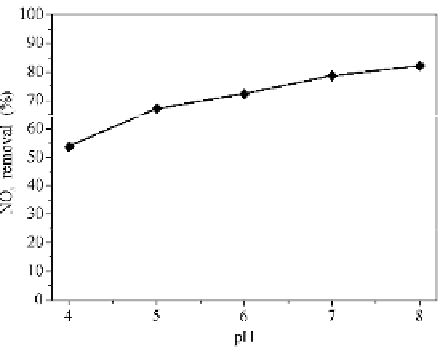
present solution conditions, two equilibrium reactions occur, as shown in Eqs. (4.8)
and (4.9). An increase in the pH value means a decrease developing in the
hydrogen ion concentration. Accordingly, the two equilibrium reactions move to a
positive direction, which dissociate SO
2
⋅H
2
O into HSO
3
, and then HSO
3
into
SO
3
2
. Again, the reaction rate between NO
2
and SO
3
2
(Eq. (4.10)) is 40 times
[24]
of that between NO
2
and HSO
3
(Eq. (4.11)). Consequently, the increasing factor
of absorption reactions (i.e.,
in Eq. (4.1)) increases, resulting a higher NO
2
removal efficiency. The pH influence on the NO
2
removal, in essence, is attributed
to the concentration changes of the tetravalent-S components such as SO
2
⋅H
2
O,
SO
3
2
, and HSO
3
with respect to pH.
HSO
3
+H
+
(4.8)
SO
2
⋅
H
2
O
=
HSO
3
=
SO
3
2
+H
+
(4.9)
2NO
2
+H
2
O+SO
3
2
=
2NO
2
+2H
+
+SO
4
2
(4.10)
2NO
2
+H
2
O+HSO
3
=
2NO
2
+3H
+
+SO
4
2
(4.11)
Fig. 4.8
Influence of the pH value on NO
2
removal with 0.01 mol/L S(IV) existence
Fig. 4.9 shows ion concentrations in the absorption solution. Concentrations of
NO
2
and NO
3
gradually increases and decreases, respectively, as the pH value
increases. This implies that SO
3
2
, which is prone to react with NO
2
, develops an
increase in its concentration, whereas the hydrolysis reaction (generating a low
NO
3
concentration) weakens to some extent. Consequently, reactions of NO
2
with
the tetravalent-S components strengthen, leading to an increasing contribution of
the redox reactions in the total NO
2
removal.
Search WWH ::

Custom Search