Environmental Engineering Reference
In-Depth Information
Fig. 4.3 shows that a low NO
2
removal efficiency of around 17% appears at
each pH setting. Similarly, Thomas and Vanderschuren
[18]
employed a padding
tower with an alkali solution to absorb NO
x
and also obtained a low removal
efficiency of 12.9%. The low removal efficiency can be attributed to the low
solubility coefficient of NO
2
. According to the two-membrane mass transfer
theory for gas and the liquid phases, the gas phase can reach a balance at the
gas-liquid interface. A low NO
2
solubility coefficient develops a low NO
2
concentration at the liquid side of the interface, thereby declining the total mass
transfer coefficient. Again, the reaction between NO
2
and water (shown in Eq.
(4.4)) associated with the NO
2
concentration, is a two-step reaction with a
relatively low reaction rate and limited increase in the NO
2
removal. Fig. 4.3 also
uncovers that an increase in the pH value leads to a nearly constant NO
2
removal
efficiency, which agrees well with the finding in the published work
[19]
. Patently,
this result denotes that no connection exists between the pH value and the removal
of NO
2
and N
2
O
4
. This observation can be mainly attributed to the fact that the
hydrolysis reaction of NO
2
in the solution is hardly controlled by the pH value.
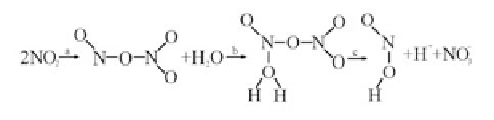
The hydrolysis reaction mechanism of NO
2
can be definitely expressed as Eq.
(4.5)
[20]
. Firstly, two NO
2
molecules produce ON-O-NO
2
through polymerization,
isomerization, and reaction in Step a. Secondly, ON-O-NO
2
is combined with a
H
2
O molecule in Step b to form an intermediate. Thirdly, the intermediate is
decomposed to HNO
2
, H
+
, and NO
3
in Step c. Among the three reaction steps,
Step a, which is not totally controlled by the pH value, is the main factor that
decides the reaction rate.
2NO
2
+H
2
O
=
HNO
3
+HNO
2
(4.4)
+
(4.5)
Fig. 4.4 shows the product concentration with respect to pH. It was found that
the main N-components in the solution after adsorbing NO
2
were NO
2
and NO
3
.
This observation fits well with the reaction in Eq. (4.4) and obviously, the molar
ratio between the two N-contained products should be theoretically 1:1. Results in
this figure also show that a slight molar concentration difference appears between
the two N-contained ions and this difference decreases with pH. In comparison

Search WWH ::

Custom Search