Environmental Engineering Reference
In-Depth Information
100
SO
2
80
60
40
20
0
0
100
200
300
400
Temperature (
ć
)
Fig. 3.13
Oxidize experimental results between O
3
and SO
2
with O
3
/SO
2
= 1.0
80
60
40
20
with SO
2
without SO
2
With SO
2
Without SO
2
0
0.0
0.2
0.4
0.6
0.8
1.0
Stoichiometric ratio of [O
3
]/[NO]
Fig. 3.14
Concentration variation in NO/O
3
reaction system after adding SO
2
(
=100 °C)
T
3.3.4 Oxidation Mechanism Between O
3
and Hg
Oxidized mercury (Hg
2+
) can be easily trapped through WFGD, but removal of the
elemental mercury (Hg
0
) proves to be challenging. Although the proportion of
each mercury species in gas phase varies with combustion conditions, coal types,
and other factors, the absorption or oxidation of elemental mercury is still the key
steps in improving the mercury removal efficiency in flue gas. Therefore, various
types of reagents and methods have been developed to promote the elemental
mercury oxidation. Apart from the oxidation of NO, O
3
and O can also efficiently
convert Hg
0
into Hg
2+
, as shown in the following reactions:
































































































































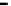













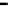

Search WWH ::

Custom Search