Environmental Engineering Reference
In-Depth Information
O
3
+NO
2
=
O
2
+NO
3
(3.7)
O
3
+O
=
2O
2
(3.8)
NO
3
+Hg
=
HgO+NO
2
(3.9)
Hg+Cl+M
HgCl+M (3.10)
HgCl+Cl
2
=
HgCl
2
+Cl (3.11)
Cl+Cl
=
Cl
2
(3.12)
Cl+OH
=
O+HCl (3.13)
=
l
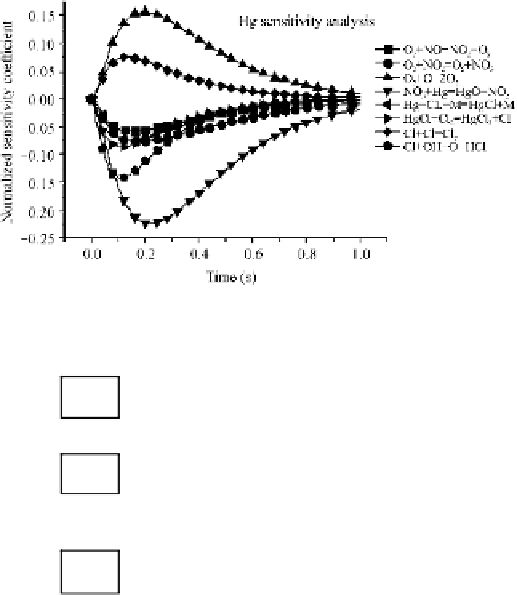
Fig. 3.6
Dimensionless sensitivity coefficient of Hg
HCl
Cl
Cl
2
Hg
HgCl
HgCl
2
HgO
O
3
NO
2
NO
3
Fig. 3.7
Oxidation path of Hg
It should be noted that the above chemical reactions are reversible thoroughly.
The foregoing analysis suggests that the Hg oxidation with HCl is mainly
facilitated by Cl (this species generated by HCl and O free radicals) and the
further conversion from HgCl to HgCl
2
essentially needs the Cl
2
assistance. This
means that compared with HCl, the mercury oxidation is more sensitive to Cl
2
when adding Cl
2
into flue gas. And the stronger mercury oxidation of Cl
2







Search WWH ::

Custom Search