Environmental Engineering Reference
In-Depth Information
O
3
+NO
=
NO
2
+O
2
Fig. 3.4
Dimensionless sensitivity
coefficients of NO
3
Fig. 3.3
Dimensionless sensitivity coefficients
of NO
2
O
3
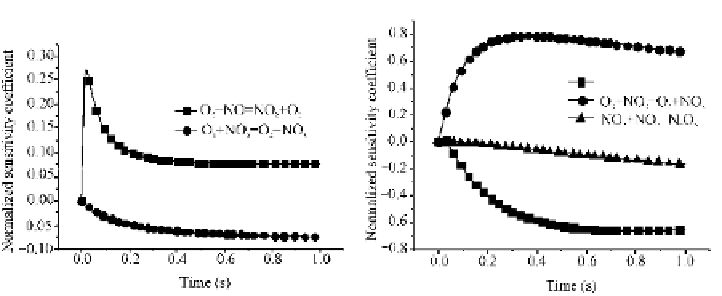
with other species are far lower than that of the mentioned elementary reaction.
Figs. 3.2 and 3.3 show that the NO and NO
2
sensitivity coefficient curves are
almost symmetrical; this observation indicates that O
3
directly oxidizing NO is a
dominant pathway that producing NO
2
. In Fig. 3.4, NO
3
is also the oxidation
product of NO
2
and O
3
. On the basis of the observations from Figs. 3.1 and 3.4, O
3
can be inferred to mainly react with NO but rarely with NO
2
when no excessive O
3
is provided. A high generation of NO
3
and N
2
O
5
appears only under the conditions
with excessive O
3
. A comprehensive consideration of the foregoing analysis about
Figs. 3.1 - 3.4 discloses the NO major oxidation pathway, as illustrated in Fig. 3.5.
The arrow thickness in Fig. 3.5 describes the reaction intensity of the branch-chain
reaction. Definitely, NO
3
and N
2
O
5
are the senior states of NO oxidation reaction,
which are generated via a step-by-sep oxidation of NO and only appear when O
3
is
excessive. Additionally, the reaction of NO with HO
2
is able to generate HNO and
then the HNO production is converted into N
2
O.
The aforementioned sensitivity analysis determines several important
elementary reactions, which are listed in a descending order of importance.
O
3
+NO
=
NO
2
+O
2
(3.1)
O
3
+NO
2
=
O
2
+NO
3
(3.2)
NO
2
+NO
3
=
N
2
O
5
(3.3)
NO+HO
2
=
NO
2
+OH (3.4)
NO+HO
2
=
HNO
3
(3.5)
Search WWH ::

Custom Search