Environmental Engineering Reference
In-Depth Information
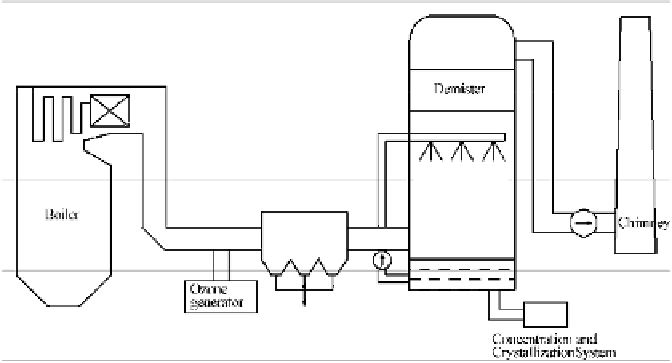
Incorporated with Ozone Oxidization and Alkali Solution Adsorption
integrated with the absorbability of acid gas of SO
2
, HCl, and HF in the alkali
solution scrubber. Afterwards, the produced sulfates and nitrates can be extracted
and concentrated in the crystallization system and then be sold as industrial raw
materials. Meanwhile, Hg
2+
in the solution can transform into the steady HgS
precipitation through the treatment of H
2
S gas, thereby avoiding secondary
pollution.
Washing
tower
Electrostatic
precipitator
Concentration and
crystallization system
Fig. 5.1
Schematic diagram of the simultaneous removal of multi-pollutants by ozone oxidation
Although here the sole denitration retrofit cost is relatively higher than any
other NO
x
reduction technologies (typically SCR and SNCR), the present
multi-pollutants removal technology focuses on the most effective removal of NO
x
,
SO
2
, Hg
0
, HCl, and HF, thereby being acknowledged as a cost-effective, near-zero
pollutant emission, co-product reutilization alternative to the development of new
multi-pollutants removal technologies. According to our experimental results, the
removal efficiencies are over 90% for NO
x
, above 99% for SO
2
, about 95% for Hg,
and over 95% for both HCl and HF. Here the NO
x
removal efficiency is also
related with combustion control in the furnace. NO
x
should be first lowered
through approaches such as low-NO
x
burners, air-staging combustion, and fuel
reburning. For example, with the initial NO
x
concentration in the furnace lowered
by about 150 - 200 ppm, the original NO
x
emissions of 800 mg/m
3
(at 6% O
2
) can
be lowered to 300 - 400 mg/m
3
(at 6% O
2
). Subsequently, another 100 - 150 ppm
reduction extent in the remaining NO
x
is achieved through ozone oxidation in flue
gas, finally resulting in a total NO
x
removal efficiency of 93.7%.

Search WWH ::

Custom Search