Biology Reference
In-Depth Information
250
200
150
100
50
0
XCC
At
Fig. 2.1
Induction of
PR1
gene in
Arabidopsis
after treatment with
Xanthomonas campestris
pv.
campestris
(
Xcc
) and
Agrobacterium tumefaciens
(
At
) muropeptides of peptidoglycans (Adapted
from Erbs et al.
2008
)
muropeptides derived from gram-negative peptidoglycans are more potent elicitors
than intact peptidoglycans (Erbs et al.
2008
). The Gram-positive bacteria-derived
peptidoglycan triggered immune responses and the peptidoglycan-mediated immu-
nity in
Arabidopsis
has been found to be based upon recognition of the sugar back-
bone in the peptidoglycan (Gust et al.
2007
). The purifi ed muropeptides of the
Gram-negative bacterial pathogens (
Xanthomonas campestris
pv.
campestris
and
Agrobacterium tumefaciens
) show higher elicitor activity than the peptidoglycan
preparations from those bacteria, suggesting that the PAMP epitope may reside in
the muropeptide moiety of the peptidoglycan (Erbs et al.
2008
). Peptidoglycans in
the bacterial surface may be degraded to muropeptides by host lysozyme activities.
The released muropeptides are highly mobile, while the peptidoglycan diffuses only
slowly (Erbs et al.
2008
).
The structure of muropeptides may differ in different bacterial pathogens.
Differences in the structures of
X
.
campestris
pv.
campestris
(
Xcc
) and
Agrobacterium
tumefaciens
muropeptides include the presence of a Gly residue replacing Ala in
the case of
A
.
tumefaciens
peptidoglycan and by the lack of an acetyl group in the
case of
Xcc
peptidoglycan (Erbs et al.
2008
). The differences observed in the muro-
peptides of the two pathogens would have contributed to the differences in their
elicitor activity. The elicitor activity of muropeptide of
X
.
campestris
pv.
campes-
tris
is very high when compared with that of
A
.
tumefaciens
(Fig.
2.1
, Erbs et al.
2008
). The studies suggest that structure and activity of peptidoglycans may vary
widely (Fig.
2.1
).
Peptidoglycan is associated with inner membrane and, in Gram-negative bacteria,
is shielded by the LPS-containing outermembrane. Peptidoglycans may be released
during growth process of the bacteria (Cloud-Hansen et al.
2006
). It is also suggested
that degradation of bacterial cells by host defenses may contribute to release of
peptidoglycan (Erbs et al.
2008
). Some plants possess peptidoglycan-modifying
lysozymes (Brunner et al. 1998) and these enzymes may release muropeptides which
may sense the pattern recognition receptors (PRRs) in host plants and activate the
innate immunity (Erbs et al.
2008
).
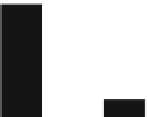










Search WWH ::

Custom Search