Agriculture Reference
In-Depth Information
IPP
MVA pathway
COOH
O
7
OPP
FDP
OH
CH
2
OH
COOH
HO
2
1
3
Botrytis cinerea
OH
COOH
O
2
E
,4
E
,6
E
-allofarnesene
ABA
OH
CH
2
OH
COOH
HO
4
5
6
Cercospora cruenta
Fig. 2.1
Proposed biosynthetic pathways of ABA in fungi. The direct pathway starts with
FDP produced from IPP via the MVA pathway.
Solid arrows
indicate one-step modifica-
tion of an intermediate and
dashed arrows
represent multistep modifications of an inter-
mediate. Abbreviations:
IPP
isopentenyl pyrophosphate,
FDP
Farnesyl diphosphate,
1
2
Z
,4
E
-
ʱ
-ionylideneethane,
2
2
Z
,4
E
-
ʱ
-ionylideneethanol,
3
1′,4′-
t
-diol-ABA,
4
2
Z
,4
E
-
ʳ
-
ionylideneethane,
5
2
Z
,4
E
-
ʳ
-ionylideneethanol,
6
2
Z
,4
E
-1′,4′-Dihyrdoxy-
ʳ
-ionylideneacetic
acid,
7
1′-deoxy ABA
indicate that ABA is synthesized by the direct pathway via the isomerization and
cyclization of allofarnesene and several oxidation steps of ionylideneethanes in
these fungi (Fig.
2.1
). After the synthesis of ionylideneethanes, ionylideneetha-
nol is supposed to be generated by their oxidation (Inomata et al.
2004a
,
b
). In
C. rosicola
,
ʱ
-ionylideneethanol and
ʱ
-ionylideneacetic acid were converted to
ABA and 1′-deoxy ABA (Fig.
2.1
) (Neill and Horgan
1983
). 1′-deoxy ABA is
thought to be the precursor of ABA in this fungus, because it is also oxidized to
ABA (Fig.
2.1
) (Neill et al.
1982
). On the one hand, in
B. cinerea
and
C. pini
-
densiflorae,
, 1′,4′-trans-diol ABA is likely the predominant precursor whose
endogenous levels are correlated with ABA synthesis (Fig.
2.1
) (Hirai et al.
1986
;
Okamoto et al.
1988
). On the other hand, 1′,4′-trans-dihydro-
ʳ
-ionylideneacetic
acid is thought to be the intermediate of ABA biosynthesis in
C. cruenta
(Fig.
2.1
)
(Oritani et al.
1985
). During the conversion from ionylideneethanes to ABA,
atmospheric oxygen is incorporated into ABA at C-1, -1, C-1′, and C-4′ in
C. cru-
enta
and
B. cinerea
(Inomata et al.
2004a
,
b
). P450 was supposed to be involved
in these oxidation steps since P450 inhibitors could effectively block ABA pro-
duction (Norman et al.
1986
). Based on this notion, Siewers et al. (
2004
) tried to
knock out two genes in
Botrytis cinerea,
BcCPR1
and
BcABA1
, encoding P450
oxidoreductase and P450, respectively. The ABA level in the
Bccpr1
mutant was
reduced compared with the wild type. ABA production was completely abolished
in the
Bcaba1
mutant (Siewers et al.
2004
). The same group also found three addi-
tional genes located around the
BcABA1
gene on the genome (Siewers et al.
2006
).
These genes were named
BcABA2
,
BcABA3,
and
BcABA4
and encoded P450,
an unknown protein and a short-chain dehydrogenase/reductase, respectively
(Siewers et al.
2006
). ABA was undetectable in the
Bcaba3
mutant but not in

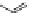










































































































Search WWH ::

Custom Search