Agriculture Reference
In-Depth Information
(a)
(b)
S
-
1
R
-
1
helix I
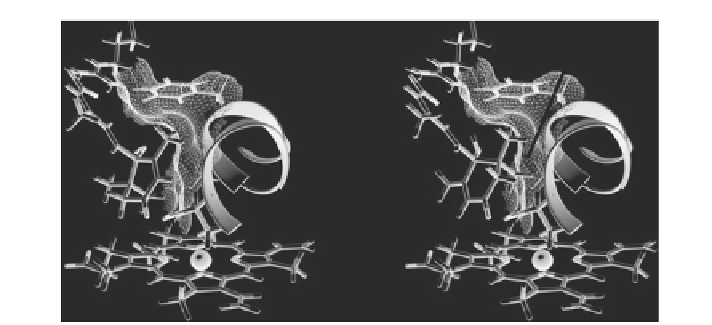
Fig. 1.13
Molecular surfaces in the active site of the 3D structural model of CYP707A3.
a
The
S
-ABA bound active site, and
b
R
-ABA superimposed and overlaid onto
S
-ABA (omitted for
simplicity)
the C-2′ side of the ring must be close to helix I (SRS 4) to keep C-8′ in close
proximity to the heme iron. If
R
-ABA binds in the same manner as
S
-ABA, C-8′
should contact helix I (Fig.
1.13
). Determination of the exact mechanism requires
the crystal structure of CYP707A, which has yet to be obtained. Another large
difference is that the side chain methyl (C-6) and C-1′ hydroxy groups which
are required for the high ABA activity is dispensable for binding to CYP707A
enzymes (Ueno et al.
2005
) (Fig.
1.14
). Focusing on this difference, Ueno et al.
(
2005
) developed AHI1 (
50
), an ABA analogue which functions as a competitive
inhibitor of CYP707A without causing an ABA response.
$
2+
2+
&2
+
&2
+
%
2
6
5HTXLUHGIRU
&<3$LQKLELWLRQ
$%$DFLWLYW\
$
&+
+
&+
%
2+
+
)
2+
Fig. 1.14
Differences in structural requirements for CYP707A3 inhibition and for ABA activity,
and AHI1, a CYP707A3 inhibitor lacking ABA activity














Search WWH ::

Custom Search