Agriculture Reference
In-Depth Information
O
H
O
H
O
O
O
H
O
10
16
OH
OH
OH
OH
OH
CO
2
H
CO
2
H
O
O
O
O
11
17
21
OH
OH
OH
β
-
D
-glucose
CO
2
H
CO
2
H
O
O
O
O
O
S
-
1
18
R
-
1
H
O
O
H
HO
OH
OH
OH
CO
2
H
CO
2
H
CO
2
H
O
O
O
12
22
19
OH
OH
OH
O
O
O
CO
2
H
CO
2
H
CO
2
H
O
O
O
13
20
23
+
OH
OH
O
O
CO
2
H
CO
2
H
HO
HO
14
15
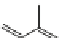
Fig. 1.6
Biosynthetic and catabolic pathway of ABA from xanthoxin. The pathway shown with
bold arrows
is the main metabolic pathway found commonly in many higher plants
7′-hydroxy-ABA (
17
), and ABA glucose ester (ABA-GE,
18
) (Ref) (Fig.
1.6
).
9′-Hydroxy-ABA (
19
) and neoPA (
20
) are found in some plants, including
Arabidopsis (Zhou et al.
2004
; Okamoto et al.
2011
). 8′-Hydroxy-ABA (
12
),
which is produced by the hydroxylation of C-8′ by CYP707A enzymes, is ther-
modynamically unstable and spontaneously isomerizes to the more stable tau-
tomer, PA. Thus, 8′-hydroxy-ABA is not stably maintained in the absence of
8′-
O
-protection following isolation. Zou et al. (
1995
) isolated 8′-hydroxy-ABA as
a borate complex by heating PA and boric acid in glacial acetic acid. The isomeri-
zation of 8′-hydroxy-ABA to PA is an intramolecular Michael-type reaction which
is accelerated under basic conditions. At 25 °C, the half-life of 8′-hydroxy-ABA
is 30 h at pH 3, 4 h at pH 7, and shorter than 1 min at pH 10 (Todoroki and Hirai
2000a
,
b
). 8′-Hydroxy-ABA is observed upon HPLC analysis of enzyme assay






































































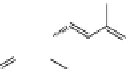


















































Search WWH ::

Custom Search