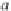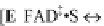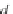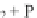Biology Reference
In-Depth Information
a
b
Fig. 11.19 A complete mechanism of the cholesterol redox cycle based on the
generalized
Franck-Condon principle
(Sect.
2.2.3
) and
the principle of microscopic reversibility
(Sect.
3.3
)
.
The
hyphen
and the
dot
symbolize noncovalent binding interactions. The superscript
*
indicates
thermally activated/excited (
b, g, j,
and
o
) or energized (
c, f, k,
and
n
) states of the enzyme-ligand
complex and the superscript
{
denotes the transition state (also called the Franck-Condon state;
Reynolds and Lumry 1966; Ji 1979). (a) The reduction of the cholesterol oxidase molecule by
cholesterol (S).
a
the oxidized enzyme in
a
thermally activated/excited state
(or a
thermally activated conformer
; see Sect.
11.3.2
);
c
¼
the oxidized enzyme before binding cholesterol;
b
¼
the
energized state of the oxidized enzyme that has bound substrate S (thermal energy is thought to be
transduced to mechanical energy upon binding S; see Sect.
11.3.2
);
d
¼
the Franck-Condon state
of the oxidized enzyme-substrate complex accessible from
c
through another round of thermal
fluctuations;
e
¼
the Franck-Condon state of the reduced enzyme-product complex thermally
accessible from
f
;
f
¼
¼
the energized state of the reduced enzyme binding product P;
g
¼
the
reduced enzyme in a thermally activated state;
h
after the product dissociates from reduced
COx. (b) The oxidation of reduced cholesterol oxidase by molecular oxygen.
i
¼
¼
the COx in
the reduced state before binding oxygen;
j
¼
the reduced COx in a thermally activated state;
k
¼
the energized COx in its reduced state binding oxygen;
l
¼
the Franck-Condon state of the
reduced COx-oxygen complex;
m
¼
the Franck-Condon state of the oxidized COx-hydrogen
peroxide complex;
n
the energized COx in its oxidized state binding hydrogen peroxide;
o ¼
the oxidized COx in a thermally activated state;
p ¼
after hydrogen peroxide dissociates
from the ground-state COx molecule
¼
performing work either on their environment (as in ion pumping or movement of
actin filament) or internally (e.g., modulation of the activation free energy barrier
for catalysis, or k
2
, in Scheme 11.17), the latter contributing to the dynamic disorder
of the COx enzymic activity.
In constructing Table
11.7
, the following assumptions have been made:
1. Both FAD and FADH
2
fluoresce, although the former is much more fluorescent
than the latter probably by a factor of about 100.
2. Both FAD and FADH
2
molecules undergo many cycles of the photon absorp-
tion-radiative decay process during the lifetime of any of the four conforma-
tional states (or conformers) of COx labeled A, B, C, and D (see Fig.
11.20
).